Bioengineered MSCCxcr2 transdifferentiated keratinocyte-like cell-derived organoid potentiates skin regeneration through ERK1/2 and STAT3 signaling in diabetic wound
- PMID: 38597972
- PMCID: PMC11006766
- DOI: 10.1007/s00018-023-05057-3
Bioengineered MSCCxcr2 transdifferentiated keratinocyte-like cell-derived organoid potentiates skin regeneration through ERK1/2 and STAT3 signaling in diabetic wound
Abstract
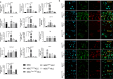
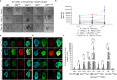
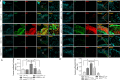
Skin regeneration is severely compromised in diabetic foot ulcers. Allogeneic mesenchymal stem cell (MSC) transplantation is limited due to the poor engraftment, mitogenic, and differentiation potential in the harsh wound microenvironment. Thus, to improve the efficacy of cell therapy, the chemokine receptor Cxcr2 was overexpressed in MSCs (MSCCxcr2). CXCL2/CXCR2 axis induction led to the enhanced proliferation of MSCs through the activation of STAT3 and ERK1/2 signaling. Transcriptional upregulation of FGFR2IIIb (KGF Receptor) promoter by the activated STAT3 and ERK1/2 suggested trans-differentiation of MSCs into keratinocytes. These stable MSCCxcr2 in 2D and 3D (spheroid) cell cultures efficiently transdifferentiated into keratinocyte-like cells (KLCs). An in vivo therapeutic potential of MSCCxcr2 transplantation and its keratinocyte-specific cell fate was observed by accelerated skin tissue regeneration in an excisional splinting wound healing murine model of streptozotocin-induced type 1 diabetes. Finally, 3D skin organoids generated using MSCCxcr2-derived KLCs upon grafting in a relatively avascular and non-healing wounds of type 2 diabetic db/db transgenic old mice resulted in a significant enhancement in the rate of wound closure by increased epithelialization (epidermal layer) and endothelialization (dermal layer). Our findings emphasize the therapeutic role of the CXCL2/CXCR2 axis in inducing trans-differentiation of the MSCs toward KLCs through the activation of ERK1/2 and STAT3 signaling and enhanced skin regeneration potential of 3D organoids grafting in chronic diabetic wounds.
Keywords: 3D skin organoids; Bioengineered MSCs; Cell transplantation; Chronic non-healing wounds; Keratinocytes; Organoid grafting; Tissue regeneration.
© 2024. The Author(s).
Conflict of interest statement
The authors have no financial or non-financial interests to disclose.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

