Predicting cytogenetic risk in multiple myeloma using conventional whole-body MRI, spinal dynamic contrast-enhanced MRI, and spinal diffusion-weighted imaging
- PMID: 38597979
- PMCID: PMC11006637
- DOI: 10.1186/s13244-024-01672-1
Predicting cytogenetic risk in multiple myeloma using conventional whole-body MRI, spinal dynamic contrast-enhanced MRI, and spinal diffusion-weighted imaging
Abstract
Objectives: Cytogenetic abnormalities are predictors of poor prognosis in multiple myeloma (MM). This paper aims to build and validate a multiparametric conventional and functional whole-body MRI-based prediction model for cytogenetic risk classification in newly diagnosed MM.
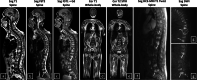
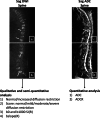
Methods: Patients with newly diagnosed MM who underwent multiparametric conventional whole-body MRI, spinal dynamic contrast-enhanced (DCE-)MRI, spinal diffusion-weighted MRI (DWI) and had genetic analysis were retrospectively included (2011-2020/Ghent University Hospital/Belgium). Patients were stratified into standard versus intermediate/high cytogenetic risk groups. After segmentation, 303 MRI features were extracted. Univariate and model-based methods were evaluated for feature and model selection. Testing was performed using receiver operating characteristic (ROC) and precision-recall curves. Models comparing the performance for genetic risk classification of the entire MRI protocol and of all MRI sequences separately were evaluated, including all features. Four final models, including only the top three most predictive features, were evaluated.
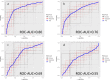
Results: Thirty-one patients were enrolled (mean age 66 ± 7 years, 15 men, 13 intermediate-/high-risk genetics). None of the univariate models and none of the models with all features included achieved good performance. The best performing model with only the three most predictive features and including all MRI sequences reached a ROC-area-under-the-curve of 0.80 and precision-recall-area-under-the-curve of 0.79. The highest statistical performance was reached when all three MRI sequences were combined (conventional whole-body MRI + DCE-MRI + DWI). Conventional MRI always outperformed the other sequences. DCE-MRI always outperformed DWI, except for specificity.
Conclusions: A multiparametric MRI-based model has a better performance in the noninvasive prediction of high-risk cytogenetics in newly diagnosed MM than conventional MRI alone.
Critical relevance statement: An elaborate multiparametric MRI-based model performs better than conventional MRI alone for the noninvasive prediction of high-risk cytogenetics in newly diagnosed multiple myeloma; this opens opportunities to assess genetic heterogeneity thus overcoming sampling bias.
Key points: • Standard genetic techniques in multiple myeloma patients suffer from sampling bias due to tumoral heterogeneity. • Multiparametric MRI noninvasively predicts genetic risk in multiple myeloma. • Combined conventional anatomical MRI, DCE-MRI, and DWI had the highest statistical performance to predict genetic risk. • Conventional MRI alone always outperformed DCE-MRI and DWI separately to predict genetic risk. DCE-MRI alone always outperformed DWI separately, except for the parameter specificity to predict genetic risk. • This multiparametric MRI-based genetic risk prediction model opens opportunities to noninvasively assess genetic heterogeneity thereby overcoming sampling bias in predicting genetic risk in multiple myeloma.
Keywords: Diffusion magnetic resonance imaging; Genetics; Magnetic resonance imaging; Multiparametric magnetic resonance imaging; Multiple myeloma.
© 2024. The Author(s).
Conflict of interest statement
Declarations of interest: the authors declare that they have no competing interests.
The authors of this manuscript declare no financial or personal relationships with any companies or individuals, whose products or services may be related to the subject matter of this article.
Figures



References
-
- Kyle RA, Durie BGM, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010 doi: 10.1038/leu.2010.60. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

