Treatment Patterns of Biologic Disease-Modifying Antirheumatic Drugs and Janus Kinase Inhibitors in Patients with Rheumatoid Arthritis in Japan: A Claims-Based Cohort Study
- PMID: 38598110
- PMCID: PMC11176134
- DOI: 10.1007/s40801-024-00423-4
Treatment Patterns of Biologic Disease-Modifying Antirheumatic Drugs and Janus Kinase Inhibitors in Patients with Rheumatoid Arthritis in Japan: A Claims-Based Cohort Study
Abstract
Background: Reports on treatment patterns of biologic disease-modifying antirheumatic drugs (bDMARDs)/Janus kinase inhibitors (JAKi) for rheumatoid arthritis (RA) in clinical practice are still sparse in Japan, especially in combination with conventional synthetic DMARDs (csDMARDs).
Objectives: The aim of this study was to investigate treatment patterns of bDMARD/JAKi in the treatment of RA in real-world clinical practice in Japan.
Method: A retrospective cohort study was conducted using the Japanese Medical Data Vision health claims database. The inclusion criteria required a recorded diagnosis of RA, defined by ICD-10 codes, in patients aged 18 years and older on the index date. We analyzed 39,903 RA patients treated with DMARDs from 2008 to 2020.
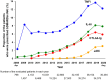
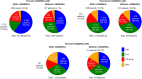
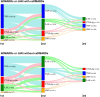
Results: Among analyzed subjects, 10,196 patients (25.6%) were prescribed bDMARDs/JAKi in combination with csDMARDs, and 3067 patients (7.7%) were prescribed these drugs without csDMARDs. Among the bDMARDs/JAKi, tumor necrosis factor inhibitors (TNFi) were the most commonly prescribed DMARD overall, and also the most common first-line therapy, accounting for 60.0% or 45.5% of patients prescribed these drugs in combination with or without csDMARDs, respectively. Switching, temporary discontinuation (restarting with the same agents), and discontinuation of bDMARDs/JAKi were observed in 3150 (30.9%), 1379 (13.5%), and 2025 (19.9%) patients with csDMARDs, and in 849 (27.7%), 513 (16.7%), and 833 (27.2%) patients without csDMARDs, respectively.
Conclusions: Real-world treatment trajectories of bDMARDs/JAKi with and without csDMARDs was analyzed in RA patients in Japan between 2008 and 2020. TNFi were the predominant first-line therapy, and likely to be switched to different classes. Understanding the current treatment patterns, including discontinuation, is important to find an optimal treatment strategy for RA patients.
© 2024. The Author(s).
Conflict of interest statement
Masahiko Miyashiro and Hirohito Shimizu are employees of Janssen Pharma K.K., a wholly owned subsidiary of Johnson & Johnson. Teita Asano, Celine Miyazaki, Yutaka Ishii, and Junya Masuda are employees of Janssen Pharmaceutical K.K., a wholly owned subsidiary of Johnson & Johnson, and may hold stock and/or stock options in the company.
Figures
Similar articles
-
Comparative effectiveness of antitumour necrosis factor agents, biologics with an alternative mode of action and tofacitinib in an observational cohort of patients with rheumatoid arthritis in Switzerland.RMD Open. 2020 May;6(1):e001174. doi: 10.1136/rmdopen-2020-001174. RMD Open. 2020. PMID: 32385143 Free PMC article.
-
Real-world treatment persistence in csDMARD-IR and bDMARD-IR patients with rheumatoid arthritis in Japan: A large claims database study.Mod Rheumatol. 2025 Jul 5;35(4):626-636. doi: 10.1093/mr/roaf007. Mod Rheumatol. 2025. PMID: 39994988
-
Real-world Comparative Effectiveness of Methotrexate-based Combinations for Rheumatoid Arthritis: A Retrospective Cohort Study.Clin Ther. 2023 Sep;45(9):e177-e186. doi: 10.1016/j.clinthera.2023.06.024. Epub 2023 Aug 10. Clin Ther. 2023. PMID: 37573225
-
Real-world effectiveness of biological therapy in patients with rheumatoid arthritis: Systematic review and meta-analysis.Front Pharmacol. 2022 Aug 11;13:927179. doi: 10.3389/fphar.2022.927179. eCollection 2022. Front Pharmacol. 2022. PMID: 36034836 Free PMC article.
-
How to Use Janus Kinase Inhibitors in the Treatment of Rheumatoid Arthritis? A Clinical Assessment of Risks and Benefits.Curr Rheumatol Rep. 2023 Dec;25(12):295-306. doi: 10.1007/s11926-023-01122-9. Epub 2023 Dec 16. Curr Rheumatol Rep. 2023. PMID: 38102522 Review.
References
LinkOut - more resources
Full Text Sources

