Bone canonical Wnt signaling is downregulated in type 2 diabetes and associates with higher advanced glycation end-products (AGEs) content and reduced bone strength
- PMID: 38598270
- PMCID: PMC11006415
- DOI: 10.7554/eLife.90437
Bone canonical Wnt signaling is downregulated in type 2 diabetes and associates with higher advanced glycation end-products (AGEs) content and reduced bone strength
Abstract
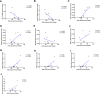
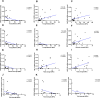
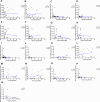
Type 2 diabetes (T2D) is associated with higher fracture risk, despite normal or high bone mineral density. We reported that bone formation genes (SOST and RUNX2) and advanced glycation end-products (AGEs) were impaired in T2D. We investigated Wnt signaling regulation and its association with AGEs accumulation and bone strength in T2D from bone tissue of 15 T2D and 21 non-diabetic postmenopausal women undergoing hip arthroplasty. Bone histomorphometry revealed a trend of low mineralized volume in T2D (T2D 0.249% [0.156-0.366]) vs non-diabetic subjects 0.352% [0.269-0.454]; p=0.053, as well as reduced bone strength (T2D 21.60 MPa [13.46-30.10] vs non-diabetic subjects 76.24 MPa [26.81-132.9]; p=0.002). We also showed that gene expression of Wnt agonists LEF-1 (p=0.0136) and WNT10B (p=0.0302) were lower in T2D. Conversely, gene expression of WNT5A (p=0.0232), SOST (p<0.0001), and GSK3B (p=0.0456) were higher, while collagen (COL1A1) was lower in T2D (p=0.0482). AGEs content was associated with SOST and WNT5A (r=0.9231, p<0.0001; r=0.6751, p=0.0322), but inversely correlated with LEF-1 and COL1A1 (r=-0.7500, p=0.0255; r=-0.9762, p=0.0004). SOST was associated with glycemic control and disease duration (r=0.4846, p=0.0043; r=0.7107, p=0.00174), whereas WNT5A and GSK3B were only correlated with glycemic control (r=0.5589, p=0.0037; r=0.4901, p=0.0051). Finally, Young's modulus was negatively correlated with SOST (r=-0.5675, p=0.0011), AXIN2 (r=-0.5523, p=0.0042), and SFRP5 (r=-0.4442, p=0.0437), while positively correlated with LEF-1 (r=0.4116, p=0.0295) and WNT10B (r=0.6697, p=0.0001). These findings suggest that Wnt signaling and AGEs could be the main determinants of bone fragility in T2D.
Keywords: AGEs; Wnt signaling; biochemistry; bone; chemical biology; diabetes; histomorphometry; human; medicine.
Plain language summary
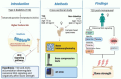
Type 2 diabetes is a long-term metabolic disease characterised by chronic high blood sugar levels. This in turn has a negative impact on the health of other tissues and organs, including bones. Type 2 diabetes patients have an increased risk of fracturing bones compared to non-diabetics. This is particularly true for fragility fractures, which are fractures caused by falls from a short height (i.e., standing height or less), often affecting hips or wrists. Usually, a lower bone density is associated with higher risk of fractures. However, patients with type 2 diabetes have increased bone fragility despite normal or higher bone density. One reason for this could be the chronically high levels of blood sugar in type 2 diabetes, which alter the properties of proteins in the body. It has been shown that the excess sugar molecules effectively ‘react’ with many different proteins, producing harmful compounds in the process, called Advanced Glycation End-products, or AGEs. AGEs are – in turn –thought to affect the structure of collagen proteins, which help hold our tissues together and decrease bone strength. However, the signalling pathways underlying this process are still unclear. To find out more, Leanza et al. studied a signalling molecule, called sclerostin, which inhibits a signalling pathway that regulates bone formation, known as Wnt signaling. The researchers compared bone samples from both diabetic and non-diabetic patients, who had undergone hip replacement surgery. Analyses of the samples, using a technique called real-time-PCR, revealed that gene expression of sclerostin was increased in samples of type 2 diabetes patients, which led to a downregulation of Wnt signaling related genes. Moreover, the downregulation of Wnt genes was correlated with lower bone strength (which was measured by compressing the bone tissue). Further biochemical analysis of the samples revealed that higher sclerostin activity was also associated with higher levels of AGEs. These results provide a clearer understanding of the biological mechanisms behind compromised bone strength in diabetes. In the future, Leanza et al. hope that this knowledge will help us develop treatments to reduce the risk of bone complications for type 2 diabetes patients.
© 2023, Leanza et al.
Conflict of interest statement
GL, FC, MF, CP, VV, FT, NP, GV, AP, RS, FZ, AB, ES, ST, RC, MM, RP, NN No competing interests declared
Figures




Update of
- doi: 10.1101/2023.10.06.23296647
- doi: 10.7554/eLife.90437.1
- doi: 10.7554/eLife.90437.2
References
-
- Andrade VFC, Chula DC, Sabbag FP, da Cavalheiro DS, Bavia L, Ambrósio AR, da Costa CRV, Dos Reis LM, Borba VZC, Moreira CA. Bone histomorphometry in young patients with type 2 diabetes is affected by disease control and chronic complications. The Journal of Clinical Endocrinology and Metabolism. 2020;105:dgz070. doi: 10.1210/clinem/dgz070. - DOI - PubMed
-
- Chen Y, Chen L, Huang R, Yang W, Chen S, Lin K, Liu J. Investigation for GSK3β expression in diabetic osteoporosis and negative osteogenic effects of GSK3β on bone marrow mesenchymal stem cells under a high glucose microenvironment. Biochemical and Biophysical Research Communications. 2021;534:727–733. doi: 10.1016/j.bbrc.2020.11.010. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

