WHAMM functions in kidney reabsorption and polymerizes actin to promote autophagosomal membrane closure and cargo sequestration
- PMID: 38598293
- PMCID: PMC11238085
- DOI: 10.1091/mbc.E24-01-0025
WHAMM functions in kidney reabsorption and polymerizes actin to promote autophagosomal membrane closure and cargo sequestration
Abstract
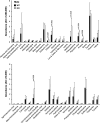
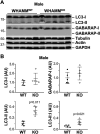
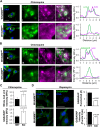
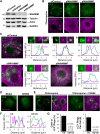
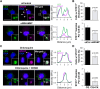
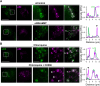
The actin cytoskeleton is essential for many functions of eukaryotic cells, but the factors that nucleate actin assembly are not well understood at the organismal level or in the context of disease. To explore the function of the actin nucleation factor WHAMM in mice, we examined how Whamm inactivation impacts kidney physiology and cellular proteostasis. We show that male WHAMM knockout mice excrete elevated levels of albumin, glucose, phosphate, and amino acids, and display structural abnormalities of the kidney proximal tubule, suggesting that WHAMM activity is important for nutrient reabsorption. In kidney tissue, the loss of WHAMM results in the accumulation of the lipidated autophagosomal membrane protein LC3, indicating an alteration in autophagy. In mouse fibroblasts and human proximal tubule cells, WHAMM and its binding partner the Arp2/3 complex control autophagic membrane closure and cargo receptor recruitment. These results reveal a role for WHAMM-mediated actin assembly in maintaining kidney function and promoting proper autophagosome membrane remodeling.
Figures



Update of
-
WHAMM functions in kidney reabsorption and polymerizes actin to promote autophagosomal membrane closure and cargo sequestration.bioRxiv [Preprint]. 2024 Jan 23:2024.01.22.576497. doi: 10.1101/2024.01.22.576497. bioRxiv. 2024. Update in: Mol Biol Cell. 2024 Jun 1;35(6):ar80. doi: 10.1091/mbc.E24-01-0025. PMID: 38328079 Free PMC article. Updated. Preprint.
References
-
- Ballabio A, Bonifacino JS (2020). Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat Rev Mol Cell Biol 21, 101–118. - PubMed
-
- Ben-Omran T, Fahiminiya S, Sorfazlian N, Almuriekhi M, Nawaz Z, Nadaf J, Khadija KA, Zaineddin S, Kamel H, Majewski J, et al. (2015). Nonsense mutation in the WDR73 gene is associated with Galloway-Mowat syndrome. J Med Genet 52, 381–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

