Modeling of brain efflux: Constraints of brain surfaces
- PMID: 38598340
- PMCID: PMC11032467
- DOI: 10.1073/pnas.2318444121
Modeling of brain efflux: Constraints of brain surfaces
Abstract
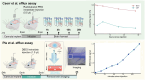
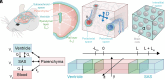
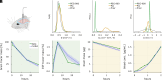
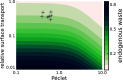
Fluid efflux from the brain plays an important role in solute waste clearance. Current experimental approaches provide little spatial information, and data collection is limited due to short duration or low frequency of sampling. One approach shows tracer efflux to be independent of molecular size, indicating bulk flow, yet also decelerating like simple membrane diffusion. In an apparent contradiction to this report, other studies point to tracer efflux acceleration. We here develop a one-dimensional advection-diffusion model to gain insight into brain efflux principles. The model is characterized by nine physiological constants and three efflux parameters for which we quantify prior uncertainty. Using Bayes' rule and the two efflux studies, we validate the model and calculate data-informed parameter distributions. The apparent contradictions in the efflux studies are resolved by brain surface boundaries being bottlenecks for efflux. To critically test the model, a custom MRI efflux assay measuring solute dispersion in tissue and release to cerebrospinal fluid was employed. The model passed the test with tissue bulk flow velocities in the range 60 to 190 [Formula: see text]m/h. Dimensional analysis identified three principal determinants of efflux, highlighting brain surfaces as a restricting factor for metabolite solute clearance.
Keywords: MRI; advection–diffusion; glymphatics.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


Similar articles
-
Human brain solute transport quantified by glymphatic MRI-informed biophysics during sleep and sleep deprivation.Fluids Barriers CNS. 2023 Aug 18;20(1):62. doi: 10.1186/s12987-023-00459-8. Fluids Barriers CNS. 2023. PMID: 37596635 Free PMC article.
-
Dispersion as a waste-clearance mechanism in flow through penetrating perivascular spaces in the brain.Sci Rep. 2021 Feb 25;11(1):4595. doi: 10.1038/s41598-021-83951-1. Sci Rep. 2021. PMID: 33633194 Free PMC article.
-
Efflux of drugs and solutes from brain: the interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters.J Cereb Blood Flow Metab. 2007 Jan;27(1):43-56. doi: 10.1038/sj.jcbfm.9600315. Epub 2006 Apr 12. J Cereb Blood Flow Metab. 2007. PMID: 16639426
-
The glymphatic hypothesis: the theory and the evidence.Fluids Barriers CNS. 2022 Feb 3;19(1):9. doi: 10.1186/s12987-021-00282-z. Fluids Barriers CNS. 2022. PMID: 35115036 Free PMC article. Review.
-
Solute transport in the brain tissue: what are the key biophysical parameters tying in vivo and in vitro studies together?Biomater Sci. 2023 May 16;11(10):3450-3460. doi: 10.1039/d3bm00027c. Biomater Sci. 2023. PMID: 37014025 Review.
Cited by
-
Estimation of fluid flow velocities in cortical brain tissue driven by the microvasculature.Interface Focus. 2025 Apr 4;15(1):20240042. doi: 10.1098/rsfs.2024.0042. eCollection 2025 Apr 4. Interface Focus. 2025. PMID: 40191021
-
Advection and diffusion in perivascular and extracellular spaces in the brain.J R Soc Interface. 2025 May;22(226):20250010. doi: 10.1098/rsif.2025.0010. Epub 2025 May 21. J R Soc Interface. 2025. PMID: 40393523 Free PMC article.
References
-
- Cserr H., Cooper D., Suri P., Patlak C., Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am. J. Physiol.-Renal Physiol. 240, F319–F328 (1981). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

