Brief parenting intervention (Triple P) for families of children with eczema: a randomized controlled trial
- PMID: 38598510
- PMCID: PMC11175588
- DOI: 10.1093/jpepsy/jsae023
Brief parenting intervention (Triple P) for families of children with eczema: a randomized controlled trial
Abstract
Objective: To evaluate the efficacy and costs of a brief, group-delivered parenting intervention for families of children with eczema.
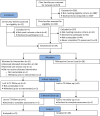
Methods: A randomized controlled trial design was used. Families attending the Queensland Children's Hospital and from the community (n = 257) were assessed for eligibility (child 2-10 years, diagnosed with eczema, prescribed topical corticosteroids). Families who consented to participate (N = 59) were assessed at baseline for clinician-rated eczema severity, parent-reported eczema symptom severity, and electronically-monitored topical corticosteroid adherence (primary outcomes); and parenting behavior, parents' self-efficacy and task performance when managing eczema, eczema-related child behavior problems, and child and parent quality of life (secondary outcomes). Families were randomized (1:1, unblinded) to intervention (n = 31) or care-as-usual (n = 28). The intervention comprised two, 2-hr Healthy Living Triple P group sessions (face-to-face/online) and 28 intervention families attended one/both sessions. All families were offered standardized eczema education. Families were reassessed at 4-weeks post-intervention and 6-month follow-up, with clinician-raters blinded to condition. Costs of intervention delivery were estimated.
Results: Multilevel modeling across assessment timepoints showed significant intervention effects for ineffective parenting (d = .60), self-efficacy (d = .74), task performance (d = .81), and confidence with managing eczema-related child behavior (d = .63), but not disease/symptom severity, treatment adherence or quality of life. Mean cost per participating family with parenting behavior (clinically) improved was $159.
Conclusions: Healthy Living Triple P is effective in reducing ineffective parenting practices and improving parents' self-efficacy and task performance when managing children's eczema and eczema-related behavior difficulties. There was no effect on disease/symptom severity, treatment adherence, or quality of life.
Clinical trial registration: ACTRN12618001332213.
Keywords: adherence/self-management; chronic illness; health behavior; parenting; parents; randomized controlled trial.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Pediatric Psychology.
Conflict of interest statement
The Parenting and Family Support Centre is partly funded by royalties stemming from published resources of the Triple P—Positive Parenting Program, which is developed and owned by The University of Queensland (UQ). Royalties are also distributed to the Faculty of Health and Behavioural Sciences at UQ and contributory authors of published Triple P resources. Triple P International (TPI) Pty Ltd is a private company licensed by Uniquest Pty Ltd on behalf of UQ, to publish and disseminate Triple P worldwide. The authors of this report have no share or ownership of TPI. Dr. Morawska receives royalties from TPI. TPI had no involvement in the study design, collection, analysis or interpretation of data, or writing of this report. Dr. Mitchell, Dr. Morawska, Ms. Johnston, and Prof Birch are employees at UQ. Ms. Forbes and Dr. Rowell were employees at UQ at the time that this research was conducted.
Figures
Similar articles
-
Effects of Triple P parenting intervention on child health outcomes for childhood asthma and eczema: Randomised controlled trial.Behav Res Ther. 2016 Aug;83:35-44. doi: 10.1016/j.brat.2016.06.001. Epub 2016 Jun 3. Behav Res Ther. 2016. PMID: 27295179 Clinical Trial.
-
Randomized controlled trial of Triple P for parents of children with asthma or eczema: Effects on parenting and child behavior.J Consult Clin Psychol. 2017 Apr;85(4):283-296. doi: 10.1037/ccp0000177. J Consult Clin Psychol. 2017. PMID: 28333531 Clinical Trial.
-
Fathers' Perceptions of Change Following Parenting Intervention: Randomized Controlled Trial of Triple P for Parents of Children With Asthma or Eczema.J Pediatr Psychol. 2017 Aug 1;42(7):792-803. doi: 10.1093/jpepsy/jsw106. J Pediatr Psychol. 2017. PMID: 28339996 Clinical Trial.
-
A video-feedback parenting intervention to prevent enduring behaviour problems in at-risk children aged 12-36 months: the Healthy Start, Happy Start RCT.Health Technol Assess. 2021 May;25(29):1-84. doi: 10.3310/hta25290. Health Technol Assess. 2021. PMID: 34018919 Free PMC article. Clinical Trial.
-
Tailoring Resources to Help Children and Parents Manage Type 1 Diabetes [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Aug. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Aug. PMID: 36701500 Free Books & Documents. Review.
Cited by
-
Acceptance and Commitment Therapy Eczema Management Program for Children With Eczema: A Pilot Randomised Controlled Trial.Clin Exp Allergy. 2025 Aug;55(8):701-715. doi: 10.1111/cea.70003. Epub 2025 Feb 3. Clin Exp Allergy. 2025. PMID: 39900107 Free PMC article. Clinical Trial.
References
-
- Arnold D. S., O’Leary S. G., Wolff L. S., Acker M. M. (1993). The Parenting Scale: A measure of dysfunctional parenting in discipline situations. Psychological Assessment, 5(2), 137–144. 10.1037/1040-3590.5.2.137 - DOI
-
- Asher M. I., Montefort S., Björkstén B., Lai C. K. W., Strachan D. P., Weiland S. K., Williams H.; ISAAC Phase Three Study Group. (2006). Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet, 368(9537), 733–743. 10.1016/S0140-6736(06)69283-0 - DOI - PubMed
-
- Australian Bureau of Statistics. (2023a). Education and work, Australia. ABS. https://www.abs.gov.au/statistics/people/education/education-and-work-au.... Date accessed January 30, 2024.
-
- Australian Bureau of Statistics. (2023b). Families and family composition. ABS. https://aifs.gov.au/research/facts-and-figures/families-and-family-compo.... Date accessed January 30, 2024.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

