Genetic interaction mapping reveals functional relationships between peptidoglycan endopeptidases and carboxypeptidases
- PMID: 38598601
- PMCID: PMC11034669
- DOI: 10.1371/journal.pgen.1011234
Genetic interaction mapping reveals functional relationships between peptidoglycan endopeptidases and carboxypeptidases
Abstract
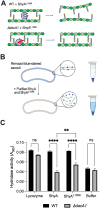
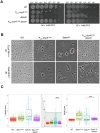
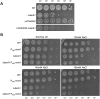
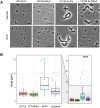
Peptidoglycan (PG) is the main component of the bacterial cell wall; it maintains cell shape while protecting the cell from internal osmotic pressure and external environmental challenges. PG synthesis is essential for bacterial growth and survival, and a series of PG modifications are required to allow expansion of the sacculus. Endopeptidases (EPs), for example, cleave the crosslinks between adjacent PG strands to allow the incorporation of newly synthesized PG. EPs are collectively essential for bacterial growth and must likely be carefully regulated to prevent sacculus degradation and cell death. However, EP regulation mechanisms are poorly understood. Here, we used TnSeq to uncover novel EP regulators in Vibrio cholerae. This screen revealed that the carboxypeptidase DacA1 (PBP5) alleviates EP toxicity. dacA1 is essential for viability on LB medium, and this essentiality was suppressed by EP overexpression, revealing that EP toxicity both mitigates, and is mitigated by, a defect in dacA1. A subsequent suppressor screen to restore viability of ΔdacA1 in LB medium identified hypomorphic mutants in the PG synthesis pathway, as well as mutations that promote EP activation. Our data thus reveal a more complex role of DacA1 in maintaining PG homeostasis than previously assumed.
Copyright: © 2024 Alvarado Obando et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Mett H, Bracha R, Mirelman D. Soluble nascent peptidoglycan in growing Escherichia coli cells. J Biol Chem. 1980. Oct 25;255(20):9884–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

