Bifurcation of Excited-State Population Leads to Anti-Kasha Luminescence in a Disulfide-Decorated Organometallic Rhenium Photosensitizer
- PMID: 38598687
- PMCID: PMC11046484
- DOI: 10.1021/jacs.4c00548
Bifurcation of Excited-State Population Leads to Anti-Kasha Luminescence in a Disulfide-Decorated Organometallic Rhenium Photosensitizer
Abstract
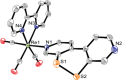
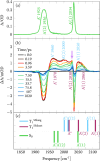
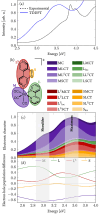
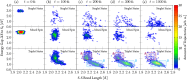
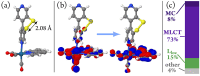
We report a rhenium diimine photosensitizer equipped with a peripheral disulfide unit on one of the bipyridine ligands, [Re(CO)3(bpy)(S-Sbpy4,4)]+ (1+, bpy = 2,2'-bipyridine, S-Sbpy4,4 = [1,2]dithiino[3,4-c:6,5-c']dipyridine), showing anti-Kasha luminescence. Steady-state and ultrafast time-resolved spectroscopies complemented by nonadiabatic dynamics simulations are used to disclose its excited-state dynamics. The calculations show that after intersystem crossing the complex evolves to two different triplet minima: a (S-Sbpy4,4)-ligand-centered excited state (3LC) lying at lower energy and a metal-to-(bpy)-ligand charge transfer (3MLCT) state at higher energy, with relative yields of 90% and 10%, respectively. The 3LC state involves local excitation of the disulfide group into the antibonding σ* orbital, leading to significant elongation of the S-S bond. Intriguingly, it is the higher-lying 3MLCT state, which is assigned to display luminescence with a lifetime of 270 ns: a signature of anti-Kasha behavior. This assignment is consistent with an energy barrier ≥ 0.6 eV or negligible electronic coupling, preventing reaction toward the 3LC state after the population is trapped in the 3MLCT state. This study represents a striking example on how elusive excited-state dynamics of transition-metal photosensitizers can be deciphered by synergistic experiments and state-of-the-art calculations. Disulfide functionalization lays the foundation of a new design strategy toward harnessing excess energy in a system for possible bimolecular electron or energy transfer reactivity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











References
-
- Crabtree G. W.; Lewis N. S. Solar energy conversion. Phys. Today 2007, 60 (3), 37–42. 10.1063/1.2718755. - DOI
-
- Blankenship R. E.; Tiede D. M.; Barber J.; Brudvig G. W.; Fleming G.; Ghirardi M.; Gunner M. R.; Junge W.; Kramer D. M.; Melis A.; Moore T. A.; Moser C. C.; Nocera D. G.; Nozik A. J.; Ort D. R.; Parson W. W.; Prince R. C.; Sayre R. T. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 2011, 332 (6031), 805–809. 10.1126/science.1200165. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

