Development of combination therapies with BTK inhibitors and dasatinib to treat CNS-infiltrating E2A-PBX1+/preBCR+ ALL
- PMID: 38598725
- PMCID: PMC11176965
- DOI: 10.1182/bloodadvances.2023011582
Development of combination therapies with BTK inhibitors and dasatinib to treat CNS-infiltrating E2A-PBX1+/preBCR+ ALL
Abstract
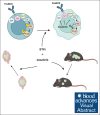
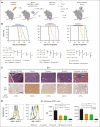
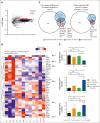
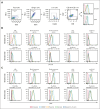
The t(1;19) translocation, encoding the oncogenic fusion protein E2A (TCF3)-PBX1, is involved in acute lymphoblastic leukemia (ALL) and associated with a pre-B-cell receptor (preBCR+) phenotype. Relapse in patients with E2A-PBX1+ ALL frequently occurs in the central nervous system (CNS). Therefore, there is a medical need for the identification of CNS active regimens for the treatment of E2A-PBX1+/preBCR+ ALL. Using unbiased short hairpin RNA (shRNA) library screening approaches, we identified Bruton tyrosine kinase (BTK) as a key gene involved in both proliferation and dasatinib sensitivity of E2A-PBX1+/preBCR+ ALL. Depletion of BTK by shRNAs resulted in decreased proliferation of dasatinib-treated E2A-PBX1+/preBCR+ cells compared with control-transduced cells. Moreover, the combination of dasatinib with BTK inhibitors (BTKi; ibrutinib, acalabrutinib, or zanubrutinib) significantly decreased E2A-PBX1+/preBCR+ human and murine cell proliferation, reduced phospholipase C gamma 2 (PLCG2) and BTK phosphorylation and total protein levels and increased disease-free survival of mice in secondary transplantation assays, particularly reducing CNS-leukemic infiltration. Hence, dasatinib with ibrutinib reduced pPLCG2 and pBTK in primary ALL patient samples, including E2A-PBX1+ ALLs. In summary, genetic depletion and pharmacological inhibition of BTK increase dasatinib effects in human and mouse with E2A-PBX1+/preBCR+ ALL across most of performed assays, with the combination of dasatinib and BTKi proving effective in reducing CNS infiltration of E2A-PBX1+/preBCR+ ALL cells in vivo.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.D.-A. received speakers honoraria from Riemser, Lilly, Ipsen, Roche, Amgen, and AstraZeneca, and travel support from AstraZeneca, Ipsen, Gilead, and Sobi. M.L. received research support from Janssen-Cilag. The remaining authors declare no competing financial interests.
Figures



References
-
- Gökbuget N, Stanze D, Beck J, et al. German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120(10):2032–2041. - PubMed
-
- Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

