An indirect comparison of acalabrutinib with and without obinutuzumab vs zanubrutinib in treatment-naive CLL
- PMID: 38598745
- PMCID: PMC11176945
- DOI: 10.1182/bloodadvances.2023012142
An indirect comparison of acalabrutinib with and without obinutuzumab vs zanubrutinib in treatment-naive CLL
Abstract
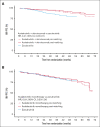
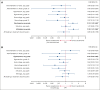
The efficacy and safety of acalabrutinib plus obinutuzumab and acalabrutinib monotherapy vs zanubrutinib in patients with treatment-naive chronic lymphocytic leukemia/small lymphocytic lymphoma without del(17p) were compared using an unanchored matching-adjusted indirect comparison. Individual patient-level data from ELEVATE-TN (acalabrutinib plus obinutuzumab, n = 162; acalabrutinib monotherapy, n = 163) were weighted to match published aggregate baseline data from SEQUOIA cohort 1, which excluded patients with del(17p) (zanubrutinib, n = 241), using variables that were prognostic/predictive of investigator-assessed progression-free survival (INV-PFS) in an exploratory Cox regression analysis of ELEVATE-TN. After matching, INV-PFS was longer with acalabrutinib plus obinutuzumab (hazard ratio [HR], 0.41; 95% confidence interval [CI], 0.23-0.74) and comparable with acalabrutinib monotherapy (HR, 0.91; 95% CI, 0.53-1.56) vs zanubrutinib. Acalabrutinib monotherapy had significantly lower odds of any grade hypertension vs zanubrutinib (odds ratio [OR], 0.44; 95% CI, 0.20-0.99), whereas acalabrutinib plus obinutuzumab had significantly higher odds of neutropenia (OR, 2.19; 95% CI, 1.33-3.60) and arthralgia (OR, 2.33; 95% CI, 1.37-3.96) vs zanubrutinib. No other significant differences in safety were observed. In summary, acalabrutinib plus obinutuzumab had longer INV-PFS with increased odds of neutropenia and arthralgia than zanubrutinib, whereas acalabrutinib monotherapy had similar INV-PFS with lower odds of any grade hypertension. These trials were registered at www.ClinicalTrials.gov as #NCT02475681 and #NCT03336333.
© 2024 by The American Society of Hematology. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
Conflict-of-interest disclosure: A.S.K. has received consulting fees from AbbVie, AstraZeneca, BeiGene, Janssen, Kite, a Gilead company, and Loxo@Lilly; has ongoing research funding from AstraZeneca and BeiGene; and is part of the speakers bureau for BeiGene. J.N.A. has received consulting fees from AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Epizyme/Ipsen, Genentech, Janssen, Lava Therapeutics, Lilly, Pharmacyclics, and TG Therapeutics; has received research funding from BeiGene, Celgene, Genentech, and Janssen; and is part of the speakers bureau for AbbVie, BeiGene, and Janssen/Pharmacyclics. D.J. has received consulting fees from AstraZeneca, United Kingdom. H.B., M.M., and F.F. are employees of AstraZeneca, United Kingdom. A.S.M.Y., J.R., and V.S. are employees of AstraZeneca, United States. H.B., M.M., A.S.M.Y., and J.R. own stocks in AstraZeneca. J.R. owns patents/royalties/other intellectual property for CalciMedica. V.S. owns stocks for Verona Pharma. A.S. holds a consulting or advisory role with AbbVie, AstraZeneca, Epizyme, Genentech, Genmab, Janssen, Kite, a Gilead company, Loxo@Lilly, MorphoSys, Novartis, Pharmacyclics, Seagen, and TG Therapeutics, and is a member of the speakers bureau for AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Celgene, Genentech, Janssen, Jazz Pharmaceuticals, Kite, a Gilead company, Loxo@Lilly, Pharmacyclics, Seagen, and TG Therapeutics. M.S.D. has received institutional research funding from AbbVie, AstraZeneca, Ascentage Pharma, Genentech, MEI Pharma, Novartis, Surface Oncology, and TG Therapeutics, and personal consulting income from AbbVie, Adaptive Biosciences, Ascentage Pharma, AstraZeneca, BeiGene, Bristol Myers Squibb, Eli Lilly, Genentech, Genmab, Janssen, Merck, MingSight Pharmaceuticals, Nuvalent, Secura Bio, TG Therapeutics, and Takeda Pharmaceuticals.
Figures

References
-
- Cho HJ, Baek DW, Kim J, Lee JM, Moon JH, Sohn SK. Keeping a balance in chronic lymphocytic leukemia (CLL) patients taking ibrutinib: ibrutinib-associated adverse events and their management based on drug interactions. Expert Rev Hematol. 2021;14(9):819–830. - PubMed
-
- Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): a covalent bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363(2):240–252. - PubMed
-
- Salem JE, Manouchehri A, Bretagne M, et al. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol. 2019;74(13):1667–1678. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

