NSD2 drives t(4;14) myeloma cell dependence on adenylate kinase 2 by diverting one-carbon metabolism to the epigenome
- PMID: 38598835
- PMCID: PMC11830969
- DOI: 10.1182/blood.2023022859
NSD2 drives t(4;14) myeloma cell dependence on adenylate kinase 2 by diverting one-carbon metabolism to the epigenome
Abstract
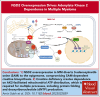
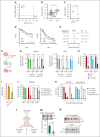
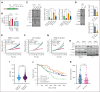
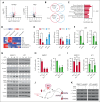
Chromosomal translocation (4;14), an adverse prognostic factor in multiple myeloma (MM), drives overexpression of the histone methyltransferase nuclear receptor binding SET domain protein 2 (NSD2). A genome-wide CRISPR screen in MM cells identified adenylate kinase 2 (AK2), an enzyme critical for high-energy phosphate transfer from the mitochondria, as an NSD2-driven vulnerability. AK2 suppression in t(4;14) MM cells decreased nicotinamide adenine dinucleotide phosphate (NADP[H]) critical for conversion of ribonucleotides to deoxyribonucleosides, leading to replication stress, DNA damage, and apoptosis. Driving a large genome-wide increase in chromatin methylation, NSD2 overexpression depletes S-adenosylmethionine, compromising the synthesis of creatine from its precursor, guanidinoacetate. Creatine supplementation restored NADP(H) levels, reduced DNA damage, and rescued AK2-deficient t(4;14) MM cells. As the creatine phosphate shuttle constitutes an alternative means for mitochondrial high-energy phosphate transport, these results indicate that NSD2-driven creatine depletion underlies the hypersensitivity of t(4;14) MM cells to AK2 loss. Furthermore, AK2 depletion in t(4;14) cells impaired protein folding in the endoplasmic reticulum, consistent with impaired use of mitochondrial adenosine triphosphate (ATP). Accordingly, AK2 suppression increased the sensitivity of MM cells to proteasome inhibition. These findings delineate a novel mechanism in which aberrant transfer of carbon to the epigenome creates a metabolic vulnerability, with direct therapeutic implications for t(4;14) MM.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-intreset disclosure: C.S.M. serves on the scientific advisory board of Adicet Bio and discloses consultant/honoraria from Genentech, Nerviano, Secura Bio and Oncopeptides, and research funding from EMD Serono, Karyopharm, Sanofi, Nurix, Bristol Myers Squibb, H3 Biomedicine/Eisai, Springworks, Abcuro, Novartis, and Opna Bio. The remaning authors declare no competing financial intresets.
Figures



Comment in
-
UnSETtling energy dependence of t(4;14) MM.Blood. 2024 Jul 18;144(3):244-245. doi: 10.1182/blood.2024024871. Blood. 2024. PMID: 39023872 No abstract available.
References
-
- Santra M, Zhan F, Tian E, Barlogie B, Shaughnessy J., Jr. A subset of multiple myeloma harboring the t(4;14)(p16;q32) translocation lacks FGFR3 expression but maintains an IGH/MMSET fusion transcript. Blood. 2003;101(6):2374–2376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

