Neuronally differentiated macula densa cells regulate tissue remodeling and regeneration in the kidney
- PMID: 38598837
- PMCID: PMC11142747
- DOI: 10.1172/JCI174558
Neuronally differentiated macula densa cells regulate tissue remodeling and regeneration in the kidney
Abstract
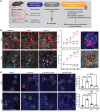
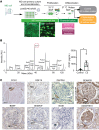
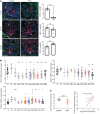
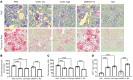
Tissue regeneration is limited in several organs, including the kidney, contributing to the high prevalence of kidney disease globally. However, evolutionary and physiological adaptive responses and the presence of renal progenitor cells suggest an existing remodeling capacity. This study uncovered endogenous tissue remodeling mechanisms in the kidney that were activated by the loss of body fluid and salt and regulated by a unique niche of a minority renal cell type called the macula densa (MD). Here, we identified neuronal differentiation features of MD cells that sense the local and systemic environment and secrete angiogenic, growth, and extracellular matrix remodeling factors, cytokines and chemokines, and control resident progenitor cells. Serial intravital imaging, MD nerve growth factor receptor and Wnt mouse models, and transcriptome analysis revealed cellular and molecular mechanisms of these MD functions. Human and therapeutic translation studies illustrated the clinical potential of MD factors, including CCN1, as a urinary biomarker and therapeutic target in chronic kidney disease. The concept that a neuronally differentiated key sensory and regulatory cell type responding to organ-specific physiological inputs controls local progenitors to remodel or repair tissues may be applicable to other organs and diverse tissue-regenerative therapeutic strategies.
Keywords: Chronic kidney disease; Nephrology.
Conflict of interest statement
Figures





Comment in
-
Remodelling by macula densa cells.Nat Rev Nephrol. 2024 Jul;20(7):429. doi: 10.1038/s41581-024-00853-x. Nat Rev Nephrol. 2024. PMID: 38816623 No abstract available.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

