Glutamatergic neuronal activity regulates angiogenesis and blood-retinal barrier maturation via Norrin/β-catenin signaling
- PMID: 38599212
- PMCID: PMC11189759
- DOI: 10.1016/j.neuron.2024.03.011
Glutamatergic neuronal activity regulates angiogenesis and blood-retinal barrier maturation via Norrin/β-catenin signaling
Abstract
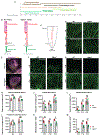
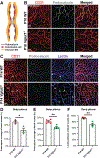
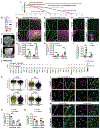
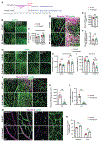
Interactions among neuronal, glial, and vascular components are crucial for retinal angiogenesis and blood-retinal barrier (BRB) maturation. Although synaptic dysfunction precedes vascular abnormalities in many retinal pathologies, how neuronal activity, specifically glutamatergic activity, regulates retinal angiogenesis and BRB maturation remains unclear. Using in vivo genetic studies in mice, single-cell RNA sequencing (scRNA-seq), and functional validation, we show that deep plexus angiogenesis and paracellular BRB maturation are delayed in Vglut1-/- retinas where neurons fail to release glutamate. By contrast, deep plexus angiogenesis and paracellular BRB maturation are accelerated in Gnat1-/- retinas, where constitutively depolarized rods release excessive glutamate. Norrin expression and endothelial Norrin/β-catenin signaling are downregulated in Vglut1-/- retinas and upregulated in Gnat1-/- retinas. Pharmacological activation of endothelial Norrin/β-catenin signaling in Vglut1-/- retinas rescues defects in deep plexus angiogenesis and paracellular BRB maturation. Our findings demonstrate that glutamatergic neuronal activity regulates retinal angiogenesis and BRB maturation by modulating endothelial Norrin/β-catenin signaling.
Keywords: Müller glia; Norrin/β-catenin signaling; angiogenesis; blood-retinal barrier; glutamate; glutamate transporter 2; neuronal activity; neurovascular development; retina; tight junctions.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Update of
-
Glutamatergic neuronal activity regulates angiogenesis and blood-retinal barrier maturation via Norrin/β-catenin signaling.bioRxiv [Preprint]. 2024 Jan 15:2023.07.10.548410. doi: 10.1101/2023.07.10.548410. bioRxiv. 2024. Update in: Neuron. 2024 Jun 19;112(12):1978-1996.e6. doi: 10.1016/j.neuron.2024.03.011. PMID: 37503079 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

