Semaglutide versus placebo in people with obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF DM randomised trials
- PMID: 38599221
- PMCID: PMC11317105
- DOI: 10.1016/S0140-6736(24)00469-0
Semaglutide versus placebo in people with obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF DM randomised trials
Abstract
Background: In the STEP-HFpEF (NCT04788511) and STEP-HFpEF DM (NCT04916470) trials, the GLP-1 receptor agonist semaglutide improved symptoms, physical limitations, bodyweight, and exercise function in people with obesity-related heart failure with preserved ejection fraction. In this prespecified pooled analysis of the STEP-HFpEF and STEP-HFpEF DM trials, we aimed to provide a more definitive assessment of the effects of semaglutide across a range of outcomes and to test whether these effects were consistent across key patient subgroups.
Methods: We conducted a prespecified pooled analysis of individual patient data from STEP-HFpEF and STEP-HFpEF DM, randomised, double-blind, placebo-controlled trials at 129 clinical research sites in 18 countries. In both trials, eligible participants were aged 18 years or older, had heart failure with a left ventricular ejection fraction of at least 45%, a BMI of at least 30 kg/m2, New York Heart Association class II-IV symptoms, and a Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS; a measure of heart failure-related symptoms and physical limitations) of less than 90 points. In STEP-HFpEF, people with diabetes or glycated haemoglobin A1c concentrations of at least 6·5% were excluded, whereas for inclusion in STEP-HFpEF DM participants had to have been diagnosed with type 2 diabetes at least 90 days before screening and to have an HbA1c of 10% or lower. In both trials, participants were randomly assigned to either 2·4 mg semaglutide once weekly or matched placebo for 52 weeks. The dual primary endpoints were change from baseline to week 52 in KCCQ-CSS and bodyweight in all randomly assigned participants. Confirmatory secondary endpoints included change from baseline to week 52 in 6-min walk distance, a hierarchical composite endpoint (all-cause death, heart failure events, and differences in changes in KCCQ-CSS and 6-min walk distance); and C-reactive protein (CRP) concentrations. Heterogeneity in treatment effects was assessed across subgroups of interest. We assessed safety in all participants who received at least one dose of study drug.
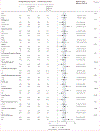
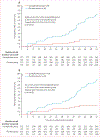
Findings: Between March 19, 2021 and March 9, 2022, 529 people were randomly assigned in STEP-HFpEF, and between June 27, 2021 and Sept 2, 2022, 616 were randomly assigned in STEP-HFpEF DM. Overall, 1145 were included in our pooled analysis, 573 in the semaglutide group and 572 in the placebo group. Improvements in KCCQ-CSS and reductions in bodyweight between baseline and week 52 were significantly greater in the semaglutide group than in the placebo group (mean between-group difference for the change from baseline to week 52 in KCCQ-CSS 7·5 points [95% CI 5·3 to 9·8]; p<0·0001; mean between-group difference in bodyweight at week 52 -8·4% [-9·2 to -7·5]; p<0·0001). For the confirmatory secondary endpoints, 6-min walk distance (mean between-group difference at week 52 17·1 metres [9·2 to 25·0]) and the hierarchical composite endpoint (win ratio 1·65 [1·42 to 1·91]) were significantly improved, and CRP concentrations (treatment ratio 0·64 [0·56 to 0·72]) were significantly reduced, in the semaglutide group compared with the placebo group (p<0·0001 for all comparisons). For the dual primary endpoints, the efficacy of semaglutide was largely consistent across multiple subgroups, including those defined by age, race, sex, BMI, systolic blood pressure, baseline CRP, and left ventricular ejection fraction. 161 serious adverse events were reported in the semaglutide group compared with 301 in the placebo group.
Interpretation: In this prespecified pooled analysis of the STEP-HFpEF and STEP-HFpEF DM trials, semaglutide was superior to placebo in improving heart failure-related symptoms and physical limitations, and reducing bodyweight in participants with obesity-related heart failure with preserved ejection fraction. These effects were largely consistent across patient demographic and clinical characteristics. Semaglutide was well tolerated.
Funding: Novo Nordisk.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests JB is a paid consultant to Abbott, American Regent, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardiac Dimension, Cardior, CVRx, Cytokinetics, Daxor Edwards, Element Science, Innolife, Impulse Dynamics, Imbria, Inventiva, Lexicon, Lilly, LivaNova, Janssen, Medtronics, Merck, Occlutech, Owkin, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Pharmain, Prolaio, Roche, Secretome, Sequana, SQ Innovation, Tenex, and Vifor. SJS has received research grants from AstraZeneca, Corvia, and Pfizer, and consulting fees from Abbott, Alleviant, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Imara, Impulse Dynamics, Intellia, Ionis, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, ReCor, Regeneron, Rivus, Sardocor, Shifamed, Tenax, Tenaya, and Ultromics. MCP has received research funding from AstraZeneca, Boehringer Ingelheim, Boston Scientific, Medtronic, Novo Nordisk, Novartis, Pharmacosmos, Roche, and SQ Innovations, and has served on committees or consulted for AbbVie, Akero, AnaCardio, Applied Therapeutics, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Cardiorentis, Corvia, Eli Lilly, Horizon Therapeutics, LIB Therapeutics, Moderna, New Amsterdam, Novartis, Novo Nordisk, Pharmacosmos, Siemens, SQ Innovations, Takeda, Teikoku, and Vifor. BAB has received research funding from AstraZeneca, Axon, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, Rivus, and Tenax Therapeutics, has served as a paid consultant for Actelion, Amgen, Aria, Axon Therapies, BD, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, NGM, Novo Nordisk, NXT, and VADovations, and is named inventor (US patent number 10 307 179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure. SZA, GKH, DVM, MNE, MLL, and SR are employees of, and shareholders in, Novo Nordisk. MJD has acted as paid consultant, advisory board member, and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi, a paid advisory board member and speaker for AstraZeneca, a paid advisory board member for Medtronic, Pfizer, and ShouTi Pharma, and a paid speaker for Amgen, Novartis, and Sanofi, and has received grants as an investigator in support of investigator-initiated trials from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, and Sanofi-Aventis. DWK reports receiving honoraria as a consultant for AstraZeneca, Bayer, Boehringer Ingelheim, Corvia Medical, Ketyo, Novartis, Novo Nordisk, Pfizer, and Rivus, has received grant funding from AstraZeneca, Bayer, Novartis, Novo Nordisk, Pfizer, and Rivus, and owns stock in Gilead. SV reports speaking honoraria or consulting fees from Abbott, Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Canadian Medical and Surgical Knowledge Translation Research Group, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, and TIMI. WA reports honoraria or consulting fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Novo Nordisk. FZA reports honoraria or consulting fees from Abbott, AstraZeneca, Medtronic, Novo Nordisk, Occlutech, Pharmacosmos, and Vifor. VC reports speaker fees from AstraZeneca, Boehringer Ingelheim, Cipla, Dr Reddy's, Lupin, Novartis, Novo Nordisk, Mankind, Pfizer, Sanofi, Sun Pharma, and Torrent. JAE reports research support for trial leadership from American Regent, Applied Therapeutics, Bayer, Cytokinetics, Merck, and Novo Nordisk, reports honoraria for consultancy from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Novo Nordisk, and Otsuka, and serves as an advisor to US2.ai. HI reports honoraria or consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Mochida, Novartis, and Novo Nordisk. ML reports honoraria or consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Ewopharma, Gedeon Richter, Novartis, Novo Nordisk, Roche, and Servier. VM reports consulting fees from Bayer, Merck Sharp & Dohme, and Novo Nordisk and research grants from Regeneron. BM reports speaker fees or research payments from Abbott, AstraZeneca, Biotronik, Boehringer Ingelheim, CSL Behring, Daiichi-Sankyo, DUKE Clinical Institute, Medtronic, and Novartis, and institutional grants from Abbott, AstraZeneca, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, CSL Behring, Daiichi-Sankyo, DUKE Clinical Institute, Eli Lilly, Medtronic, Novartis, Terumo, and Vifor. JN reports honoraria or consulting fees from Alleviant, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Pfizer, Novartis, Novo Nordisk, Rovi, and Vifor. EP reports honoraria from Novo Nordisk. MSc reports speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, and Novo Nordisk. MSe reports honoraria or consulting fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Vifor. KS received honoraria for serving as an advisory board member and consultant for Alleviant, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Janssen, Novartis, Novo Nordisk, and Rivus. PvdM reports institutional payments for consultancy fees or grants from AstraZeneca, Boehringer Ingelheim, BridgeBio, Ionis, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Pharma Nord, and Vifor. DVL reports honoraria or consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Novo Nordisk, Recardio, Sanofi, Sanova, and Vaxxinity. DW reports consultancy fees from Novo Nordisk. MNK served as a paid consultant or advisory board member for 35Pharma, Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Imbria Pharmaceuticals, Janssen, Lexicon Pharmaceuticals, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Pfizer, Sanofi, scPharmaceuticals, Structure Therapeutics, Vifor, and Youngene Therapeutics, has received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, holds stocks in Artera Health and Saghmos Therapeutics, has received honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk, and has received other research support from AstraZeneca. TB-G and MF report no competing interests.
Figures

Comment in
-
Semaglutide-a new treatment for obesity-related heart failure with preserved ejection fraction?Lancet. 2024 Apr 27;403(10437):1604-1606. doi: 10.1016/S0140-6736(24)00653-6. Epub 2024 Apr 7. Lancet. 2024. PMID: 38599218 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

