ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D
- PMID: 38599239
- PMCID: PMC11283288
- DOI: 10.1038/s41586-024-07373-5
ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D
Abstract
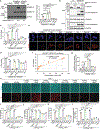
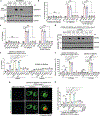
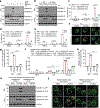
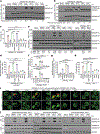
Gasdermin D (GSDMD) is the common effector for cytokine secretion and pyroptosis downstream of inflammasome activation and was previously shown to form large transmembrane pores after cleavage by inflammatory caspases to generate the GSDMD N-terminal domain (GSDMD-NT)1-10. Here we report that GSDMD Cys191 is S-palmitoylated and that palmitoylation is required for pore formation. S-palmitoylation, which does not affect GSDMD cleavage, is augmented by mitochondria-generated reactive oxygen species (ROS). Cleavage-deficient GSDMD (D275A) is also palmitoylated after inflammasome stimulation or treatment with ROS activators and causes pyroptosis, although less efficiently than palmitoylated GSDMD-NT. Palmitoylated, but not unpalmitoylated, full-length GSDMD induces liposome leakage and forms a pore similar in structure to GSDMD-NT pores shown by cryogenic electron microscopy. ZDHHC5 and ZDHHC9 are the major palmitoyltransferases that mediate GSDMD palmitoylation, and their expression is upregulated by inflammasome activation and ROS. The other human gasdermins are also palmitoylated at their N termini. These data challenge the concept that cleavage is the only trigger for GSDMD activation. They suggest that reversible palmitoylation is a checkpoint for pore formation by both GSDMD-NT and intact GSDMD that functions as a general switch for the activation of this pore-forming family.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures










Update of
-
ROS-dependent palmitoylation is an obligate licensing modification for GSDMD pore formation.bioRxiv [Preprint]. 2023 Mar 7:2023.03.07.531538. doi: 10.1101/2023.03.07.531538. bioRxiv. 2023. Update in: Nature. 2024 Jun;630(8016):437-446. doi: 10.1038/s41586-024-07373-5. PMID: 36945424 Free PMC article. Updated. Preprint.
References
-
- Kayagaki N et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). - PubMed
-
- Shi J et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). - PubMed
-
- Ding J et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016). - PubMed
-
- Gaidt MM & Hornung V The NLRP3 inflammasome renders cell death pro-inflammatory. J. Mol. Biol. 430, 133–141 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

