Utility of testing for third-generation anticyclic citrullinated peptide (anti-CCP3) antibodies in individuals who present with new musculoskeletal symptoms but have a negative second-generation anticyclic citrullinated peptide (anti-CCP2) antibody test
- PMID: 38599655
- PMCID: PMC11015229
- DOI: 10.1136/rmdopen-2023-003927
Utility of testing for third-generation anticyclic citrullinated peptide (anti-CCP3) antibodies in individuals who present with new musculoskeletal symptoms but have a negative second-generation anticyclic citrullinated peptide (anti-CCP2) antibody test
Abstract
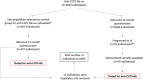
Objectives: To investigate the role of third-generation anticyclic citrullinated peptide (anti-CCP3) antibodies in predicting progression to inflammatory arthritis (IA) in individuals with new musculoskeletal (MSK) symptoms and a negative second-generation anti-CCP antibody test (anti-CCP2-).
Methods: 469 anti-CCP2- individuals underwent baseline anti-CCP3 testing (QUANTA Lite CCP3; Inova Diagnostics) and received a post enrolment 12-month questionnaire. A rheumatologist confirmed or excluded diagnosis of IA. Univariable/multivariable analyses were performed to assess the value of anti-CCP3 in predicting IA development in these anti-CCP2- individuals.
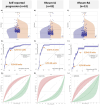
Results: Only 16/469 (3.4%) anti-CCP2- individuals had a positive anti-CCP3 test. Of these 16 individuals, 4 developed IA. In addition, 61/469 (13.0%) anti-CCP2- individuals self-reported, to have developed, IA. Progression was confirmed in 43/61 of them (70.5%); of whom 30/43 (69.8%) and 13/43 (30.2%) were given a diagnosis of IA and rheumatoid arthritis (RA), respectively. In qualitative univariable analysis, anti-CCP3 positivity was associated with self-reported progression (p<0.01) and IA (p=0.03), but not with RA. Anti-CCP3 levels differed significantly between progressors and non-progressors (p<0.01) for all three categories. At the manufacturer's cut-off, OR for progression ranged from 2.4 (95% CI 0.5 to 18.6; RA) to 7.5 (95% CI 2.3 to 24.0; self-reported progression). Interestingly, when cut-offs for anti-CCP3 were optimised, lower values (≥5 units) significantly increased the OR for progression in all three categories. In multivariable analysis, anti-CCP3 positivity at the manufacturer's cut-off did not remain associated with IA progression, while this lower cut-off value (≥5 units) was associated with diagnosis of RA (p=0.02).
Conclusions: Anti-CCP3 testing could improve the prediction of IA development in anti-CCP2- individuals with new MSK symptoms.
Keywords: Anti-Citrullinated Protein Antibodies; Antibodies; Arthritis, Rheumatoid.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ADM has received speaking fees from Janssen. KM reports personal fees from Abbvie, Lilly, Galapagos, UCB and Serac Healthcare outside the submitted work and research grants from Gilead, Serac Healthcare and Lilly. MM is employed at Werfen, a diagnostic company that commercialises the CCP3 assay. He does not have stocks or shares of the company or other incentives for the product. Testing was done at the University of Leeds and MM was not involved. PE reports providing expert advice to Abbvie, Astra-Zeneca, BMS, Boehringer Ingelheim, Galapagos, Gilead, Lilly, Novartis, Pfizer, Roche, Samsung outside the submitted work. He also reports research grants from AbbVie, BMS, Lilly and Samsung. The remaining authors have declared no conflicts of interest.
Figures

Similar articles
-
Anti-citrullinated protein antibodies in the diagnosis of rheumatoid arthritis (RA): diagnostic performance of automated anti-CCP-2 and anti-CCP-3 antibodies assays.Clin Rheumatol. 2017 Jul;36(7):1487-1492. doi: 10.1007/s10067-017-3684-8. Epub 2017 Jun 3. Clin Rheumatol. 2017. PMID: 28578492 Free PMC article.
-
Third generation anti-citrullinated peptide antibody assay is a sensitive marker in rheumatoid factor negative rheumatoid arthritis.Clin Chim Acta. 2012 Dec 24;414:266-72. doi: 10.1016/j.cca.2012.09.015. Epub 2012 Sep 25. Clin Chim Acta. 2012. PMID: 23022338
-
Third-Generation Anti-Cyclic Citrullinated Peptide Antibodies Improve Prediction of Clinical Arthritis in Individuals at Risk of Rheumatoid Arthritis.Arthritis Rheumatol. 2020 Nov;72(11):1820-1828. doi: 10.1002/art.41402. Epub 2020 Sep 7. Arthritis Rheumatol. 2020. PMID: 32840033
-
Anti-citrullinated protein antibodies in rheumatoid arthritis: as good as it gets?Clin Rev Allergy Immunol. 2008 Feb;34(1):26-31. doi: 10.1007/s12016-007-8022-5. Clin Rev Allergy Immunol. 2008. PMID: 18270854 Review.
-
Anti-citrullinated protein antibodies and their clinical utility in rheumatoid arthritis.Int J Rheum Dis. 2013 Aug;16(4):379-86. doi: 10.1111/1756-185X.12129. Epub 2013 Jul 15. Int J Rheum Dis. 2013. PMID: 23992255 Review.
Cited by
-
Biomarkers in the diagnosis, prognosis and management of rheumatoid arthritis: A comprehensive review.Ann Clin Biochem. 2025 Jan;62(1):3-21. doi: 10.1177/00045632241285843. Epub 2024 Oct 1. Ann Clin Biochem. 2025. PMID: 39242085 Free PMC article. Review.
References
-
- Verheul MK, Böhringer S, van Delft MAM, et al. . Triple positivity for anti-Citrullinated protein Autoantibodies, rheumatoid factor, and anti-Carbamylated protein antibodies conferring high specificity for rheumatoid arthritis: implications for very early identification of at-risk individuals. Arthritis Rheumatol 2018;70:1721–31. 10.1002/art.40562 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
