Transcriptional programs mediating neuronal toxicity and altered glial-neuronal signaling in a Drosophila knock-in tauopathy model
- PMID: 38599684
- PMCID: PMC11146598
- DOI: 10.1101/gr.278576.123
Transcriptional programs mediating neuronal toxicity and altered glial-neuronal signaling in a Drosophila knock-in tauopathy model
Abstract
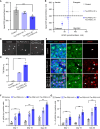
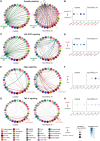
Missense mutations in the gene encoding the microtubule-associated protein TAU (current and approved symbol is MAPT) cause autosomal dominant forms of frontotemporal dementia. Multiple models of frontotemporal dementia based on transgenic expression of human TAU in experimental model organisms, including Drosophila, have been described. These models replicate key features of the human disease but do not faithfully recreate the genetic context of the human disorder. Here we use CRISPR-Cas-mediated gene editing to model frontotemporal dementia caused by the TAU P301L mutation by creating the orthologous mutation, P251L, in the endogenous Drosophila tau gene. Flies heterozygous or homozygous for Tau P251L display age-dependent neurodegeneration, display metabolic defects, and accumulate DNA damage in affected neurons. To understand the molecular events promoting neuronal dysfunction and death in knock-in flies, we performed single-cell RNA sequencing on approximately 130,000 cells from brains of Tau P251L mutant and control flies. We found that expression of disease-associated mutant tau altered gene expression cell autonomously in all neuronal cell types identified. Gene expression was also altered in glial cells, suggestive of non-cell-autonomous regulation. Cell signaling pathways, including glial-neuronal signaling, were broadly dysregulated as were brain region and cell type-specific protein interaction networks and gene regulatory programs. In summary, we present here a genetic model of tauopathy that faithfully recapitulates the genetic context and phenotypic features of the human disease, and use the results of comprehensive single-cell sequencing analysis to outline pathways of neurotoxicity and highlight the potential role of non-cell-autonomous changes in glia.
© 2024 Bukhari et al.; Published by Cold Spring Harbor Laboratory Press.
Figures






Update of
-
Transcriptional programs mediating neuronal toxicity and altered glial-neuronal signaling in a Drosophila knock-in tauopathy model.bioRxiv [Preprint]. 2024 Feb 4:2024.02.02.578624. doi: 10.1101/2024.02.02.578624. bioRxiv. 2024. Update in: Genome Res. 2024 May 15;34(4):590-605. doi: 10.1101/gr.278576.123. PMID: 38352559 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
