Fosgonimeton attenuates amyloid-beta toxicity in preclinical models of Alzheimer's disease
- PMID: 38599894
- PMCID: PMC11067346
- DOI: 10.1016/j.neurot.2024.e00350
Fosgonimeton attenuates amyloid-beta toxicity in preclinical models of Alzheimer's disease
Abstract
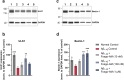
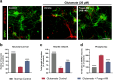
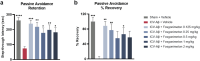
Positive modulation of hepatocyte growth factor (HGF) signaling may represent a promising therapeutic strategy for Alzheimer's disease (AD) based on its multimodal neurotrophic, neuroprotective, and anti-inflammatory effects addressing the complex pathophysiology of neurodegeneration. Fosgonimeton is a small-molecule positive modulator of the HGF system that has demonstrated neurotrophic and pro-cognitive effects in preclinical models of dementia. Herein, we evaluate the neuroprotective potential of fosgonimeton, or its active metabolite, fosgo-AM, in amyloid-beta (Aβ)-driven preclinical models of AD, providing mechanistic insight into its mode of action. In primary rat cortical neurons challenged with Aβ (Aβ1-42), fosgo-AM treatment significantly improved neuronal survival, protected neurite networks, and reduced tau hyperphosphorylation. Interrogation of intracellular events indicated that cortical neurons treated with fosgo-AM exhibited a significant decrease in mitochondrial oxidative stress and cytochrome c release. Following Aβ injury, fosgo-AM significantly enhanced activation of pro-survival effectors ERK and AKT, and reduced activity of GSK3β, one of the main kinases involved in tau hyperphosphorylation. Fosgo-AM also mitigated Aβ-induced deficits in Unc-like kinase 1 (ULK1) and Beclin-1, suggesting a potential effect on autophagy. Treatment with fosgo-AM protected cortical neurons from glutamate excitotoxicity, and such effects were abolished in the presence of an AKT or MEK/ERK inhibitor. In vivo, fosgonimeton administration led to functional improvement in an intracerebroventricular Aβ25-35 rat model of AD, as it significantly rescued cognitive function in the passive avoidance test. Together, our data demonstrate the ability of fosgonimeton to counteract mechanisms of Aβ-induced toxicity. Fosgonimeton is currently in clinical trials for mild-to-moderate AD (NCT04488419; NCT04886063).
Keywords: Alzheimer's disease; Amyloid beta; Fosgonimeton; Hepatocyte growth factor (HGF); Neuroprotection; Neurotrophic factor.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kevin J Church reports a relationship with Athira Pharma Inc that includes: employment, equity or stocks, and travel reimbursement. Sherif M Reda reports a relationship with Athira Pharma Inc that includes: employment, equity or stocks, and travel reimbursement. Sharay E Setti reports a relationship with Athira Pharma Inc that includes: employment, equity or stocks, and travel reimbursement. Andree-Anne Berthiaume reports a relationship with Athira Pharma Inc that includes: employment, equity or stocks, and travel reimbursement. Wei Wu reports a relationship with Athira Pharma Inc that includes: employment, equity or stocks, and travel reimbursement. Robert W Taylor reports a relationship with Athira Pharma Inc that includes: employment, equity or stocks, and travel reimbursement. Jewel L Johnston reports a relationship with Athira Pharma Inc that includes: employment, equity or stocks, and travel reimbursement. Liana R Stein reports a relationship with Athira Pharma Inc that includes: employment, equity or stocks, and travel reimbursement. Hans J Moebius reports a relationship with Athira Pharma Inc that includes: employment, equity or stocks, and travel reimbursement. Kevin J Church has patent issued to Athira Pharma Inc. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



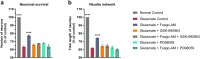
References
-
- 2022 Alzheimer's disease facts and figures. Alzheimers Dement. 2022 Apr 1;18(4):700–789. - PubMed
-
- Gate Neuroinflammation in Alzheimer's disease. J Neurol Sci. 2021 Oct 1;429
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

