Genetically encoded transcriptional plasticity underlies stress adaptation in Mycobacterium tuberculosis
- PMID: 38600064
- PMCID: PMC11006872
- DOI: 10.1038/s41467-024-47410-5
Genetically encoded transcriptional plasticity underlies stress adaptation in Mycobacterium tuberculosis
Abstract
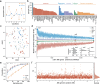
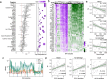
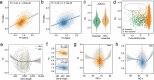
Transcriptional regulation is a critical adaptive mechanism that allows bacteria to respond to changing environments, yet the concept of transcriptional plasticity (TP) - the variability of gene expression in response to environmental changes - remains largely unexplored. In this study, we investigate the genome-wide TP profiles of Mycobacterium tuberculosis (Mtb) genes by analyzing 894 RNA sequencing samples derived from 73 different environmental conditions. Our data reveal that Mtb genes exhibit significant TP variation that correlates with gene function and gene essentiality. We also find that critical genetic features, such as gene length, GC content, and operon size independently impose constraints on TP, beyond trans-regulation. By extending our analysis to include two other Mycobacterium species -- M. smegmatis and M. abscessus -- we demonstrate a striking conservation of the TP landscape. This study provides a comprehensive understanding of the TP exhibited by mycobacteria genes, shedding light on this significant, yet understudied, genetic feature encoded in bacterial genomes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
Genetically encoded transcriptional plasticity underlies stress adaptation in Mycobacterium tuberculosis.bioRxiv [Preprint]. 2023 Aug 20:2023.08.20.553992. doi: 10.1101/2023.08.20.553992. bioRxiv. 2023. Update in: Nat Commun. 2024 Apr 10;15(1):3088. doi: 10.1038/s41467-024-47410-5. PMID: 37645742 Free PMC article. Updated. Preprint.
-
Genetically encoded transcriptional plasticity underlies stress adaptation in Mycobacterium tuberculosis.Res Sq [Preprint]. 2023 Sep 18:rs.3.rs-3303807. doi: 10.21203/rs.3.rs-3303807/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Apr 10;15(1):3088. doi: 10.1038/s41467-024-47410-5. PMID: 37790329 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

