Joint-specific memory, resident memory T cells and the rolling window of opportunity in arthritis
- PMID: 38600215
- PMCID: PMC11295581
- DOI: 10.1038/s41584-024-01107-7
Joint-specific memory, resident memory T cells and the rolling window of opportunity in arthritis
Abstract
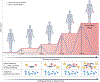
In rheumatoid arthritis, juvenile idiopathic arthritis and other forms of inflammatory arthritis, the immune system targets certain joints but not others. The pattern of joints affected varies by disease and by individual, with flares most commonly involving joints that were previously inflamed. This phenomenon, termed joint-specific memory, is difficult to explain by systemic immunity alone. Mechanisms of joint-specific memory include the involvement of synovial resident memory T cells that remain in the joint during remission and initiate localized disease recurrence. In addition, arthritis-induced durable changes in synovial fibroblasts and macrophages can amplify inflammation in a site-specific manner. Together with ongoing systemic processes that promote extension of arthritis to new joints, these local factors set the stage for a stepwise progression in disease severity, a paradigm for arthritis chronicity that we term the joint accumulation model. Although durable drug-free remission through early treatment remains elusive for most forms of arthritis, the joint accumulation paradigm defines new therapeutic targets, emphasizes the importance of sustained treatment to prevent disease extension to new joints, and identifies a rolling window of opportunity for altering the natural history of arthritis that extends well beyond the initiation phase of disease.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing interests
P.A.N. declares consulting relationships with Alkermes, Apollo, BMS, Exo Therapeutics, Fresh Tracks Therapeutics, Merck, Novartis, Pfizer, Qiagen and Sobi; equity in Edelweiss Immune Inc.; investigator-initiated research grants from BMS and Pfizer; and authorship and editorial income from UpToDate, the American Academy of Paediatrics and
Figures


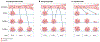
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

