Transcription-coupled repair of DNA-protein cross-links depends on CSA and CSB
- PMID: 38600235
- PMCID: PMC11098753
- DOI: 10.1038/s41556-024-01391-1
Transcription-coupled repair of DNA-protein cross-links depends on CSA and CSB
Abstract
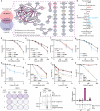
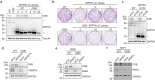
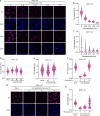
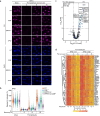
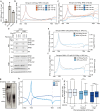
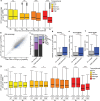
Covalent DNA-protein cross-links (DPCs) are toxic DNA lesions that block replication and require repair by multiple pathways. Whether transcription blockage contributes to the toxicity of DPCs and how cells respond when RNA polymerases stall at DPCs is unknown. Here we find that DPC formation arrests transcription and induces ubiquitylation and degradation of RNA polymerase II. Using genetic screens and a method for the genome-wide mapping of DNA-protein adducts, DPC sequencing, we discover that Cockayne syndrome (CS) proteins CSB and CSA provide resistance to DPC-inducing agents by promoting DPC repair in actively transcribed genes. Consequently, CSB- or CSA-deficient cells fail to efficiently restart transcription after induction of DPCs. In contrast, nucleotide excision repair factors that act downstream of CSB and CSA at ultraviolet light-induced DNA lesions are dispensable. Our study describes a transcription-coupled DPC repair pathway and suggests that defects in this pathway may contribute to the unique neurological features of CS.
© 2024. The Author(s).
Conflict of interest statement
S.B. is a founder and shareholder of Biomodal Ltd., Inflex Ltd. and RNAvate Ltd. L.M. is a consultant for Inflex Ltd. S.P.J works part time as Chief Research Officer at Insmed Innovation UK Ltd. S.P.J is a founding partner of Ahren Innovation Capital LLP and is co-founder, a board member and Chair of the Scientific Advisory Board of Mission Therapeutics Ltd. S.P.J. is a consultant and shareholder of Inflex Ltd. The authors declare no other competing interests.
Figures


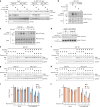
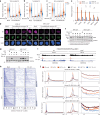
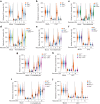
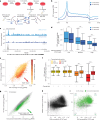




References
Publication types
MeSH terms
Substances
Grants and funding
- Discovery Award DRCPGM\100005/Cancer Research UK (CRUK)
- Vallee Scholar Award/Vallee Foundation (Bert L. & N. Kuggie Vallee Foundation)
- C9681/A29214/Cancer Research UK (CRUK)
- YIP4644/European Molecular Biology Laboratory (EMBL Heidelberg)
- 19836/CRUK_/Cancer Research UK/United Kingdom
- Project-ID 393547839 - SFB 1361/Deutsche Forschungsgemeinschaft (German Research Foundation)
- WT_/Wellcome Trust/United Kingdom
- SFB824/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 20-203-RCDA/Israel Cancer Research Fund (Israel Cancer Research Fund, Inc.)
- A:29580/Cancer Research UK (CRUK)
- 101043815/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 855741/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- CRUK RadNet grant C17918/A28870/Cancer Research UK (CRUK)
- Alfried Krupp Prize for Young University Teachers/Alfried Krupp von Bohlen und Halbach-Stiftung (Alfried Krupp von Bohlen und Halbach Foundation)
- C9545/A19836/Cancer Research UK (CRUK)
- 801750/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 482/22/Israel Science Foundation (ISF)
- CRUK Discovery Award DRCPGM\100005/Cancer Research UK (CRUK)
LinkOut - more resources
Full Text Sources
Research Materials

