Force-controlled release of small molecules with a rotaxane actuator
- PMID: 38600268
- PMCID: PMC11006608
- DOI: 10.1038/s41586-024-07154-0
Force-controlled release of small molecules with a rotaxane actuator
Abstract
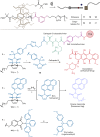
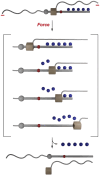
Force-controlled release of small molecules offers great promise for the delivery of drugs and the release of healing or reporting agents in a medical or materials context1-3. In polymer mechanochemistry, polymers are used as actuators to stretch mechanosensitive molecules (mechanophores)4. This technique has enabled the release of molecular cargo by rearrangement, as a direct5,6 or indirect7-10 consequence of bond scission in a mechanophore, or by dissociation of cage11, supramolecular12 or metal complexes13,14, and even by 'flex activation'15,16. However, the systems described so far are limited in the diversity and/or quantity of the molecules released per stretching event1,2. This is due to the difficulty in iteratively activating scissile mechanophores, as the actuating polymers will dissociate after the first activation. Physical encapsulation strategies can be used to deliver a larger cargo load, but these are often subject to non-specific (that is, non-mechanical) release3. Here we show that a rotaxane (an interlocked molecule in which a macrocycle is trapped on a stoppered axle) acts as an efficient actuator to trigger the release of cargo molecules appended to its axle. The release of up to five cargo molecules per rotaxane actuator was demonstrated in solution, by ultrasonication, and in bulk, by compression, achieving a release efficiency of up to 71% and 30%, respectively, which places this rotaxane device among the most efficient release systems achieved so far1. We also demonstrate the release of three representative functional molecules (a drug, a fluorescent tag and an organocatalyst), and we anticipate that a large variety of cargo molecules could be released with this device. This rotaxane actuator provides a versatile platform for various force-controlled release applications.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Willis-Fox N, Rognin E, Aljohani TA, Daly R. Polymer mechanochemistry: manufacturing is now a force to be reckoned with. Chem. 2018;4:2499–2537. doi: 10.1016/j.chempr.2018.08.001. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

