Modeling HIV-1 infection and NeuroHIV in hiPSCs-derived cerebral organoid cultures
- PMID: 38600307
- PMCID: PMC11464638
- DOI: 10.1007/s13365-024-01204-z
Modeling HIV-1 infection and NeuroHIV in hiPSCs-derived cerebral organoid cultures
Abstract
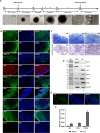
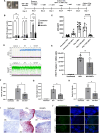
The human immunodeficiency virus (HIV) epidemic is an ongoing global health problem affecting 38 million people worldwide with nearly 1.6 million new infections every year. Despite the advent of combined antiretroviral therapy (cART), a large percentage of people with HIV (PWH) still develop neurological deficits, grouped into the term of HIV-associated neurocognitive disorders (HAND). Investigating the neuropathology of HIV is important for understanding mechanisms associated with cognitive impairment seen in PWH. The major obstacle for studying neuroHIV is the lack of suitable in vitro human culture models that could shed light into the HIV-CNS interactions. Recent advances in induced pluripotent stem cell (iPSC) culture and 3D brain organoid systems have allowed the generation of 2D and 3D culture methods that possess a potential to serve as a model of neurotropic viral diseases, including HIV. In this study, we first generated and characterized several hiPSC lines from healthy human donor skin fibroblast cells. hiPSCs were then used for the generation of microglia-containing human cerebral organoids (hCOs). Once fully characterized, hCOs were infected with HIV-1 in the presence and absence of cART regimens and viral infection was studied by cellular, molecular/biochemical, and virological assays. Our results revealed that hCOs were productively infected with HIV-1 as evident by viral p24-ELISA in culture media, RT-qPCR and RNAscope analysis of viral RNA, as well as ddPCR analysis of proviral HIV-1 in genomic DNA samples. More interestingly, replication and gene expression of HIV-1 were also greatly suppressed by cART in hCOs as early as 7 days post-infections. Our results suggest that hCOs derived from hiPSCs support HIV-1 replication and gene expression and may serve as a unique platform to better understand neuropathology of HIV infection in the brain.
Keywords: Astrocytes; Cerebral organoids; HIV-1; Microglia; NeuroHIV; cART; hiPSCs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Adamson DC, Wildemann B, Sasaki M et al (1996) Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science 274:1917–1921. 10.1126/science.274.5294.1917 - PubMed
-
- Agrawal L, Louboutin J-P, Strayer DS (2007) Preventing HIV-1 tat-induced neuronal apoptosis using antioxidant enzymes: mechanistic and therapeutic implications. Virology 363:462–472. 10.1016/j.virol.2007.02.004 - PubMed
-
- Albright AV, Shieh JT, O’Connor MJ, González-Scarano F (2000) Characterization of cultured microglia that can be infected by HIV-1. J Neurovirol 6(Suppl 1):S53–60 - PubMed
-
- Barber TJ, Imaz A, Boffito M et al (2018) CSF inflammatory markers and neurocognitive function after addition of maraviroc to monotherapy darunavir/ritonavir in stable HIV patients: the CINAMMON study. J Neurovirol 24:98–105. 10.1007/s13365-017-0600-6 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

