ECLIM-SEHOP: how to develop a platform to conduct academic trials for childhood cancer
- PMID: 38600340
- PMCID: PMC11333546
- DOI: 10.1007/s12094-024-03445-0
ECLIM-SEHOP: how to develop a platform to conduct academic trials for childhood cancer
Abstract
Introduction: ECLIM-SEHOP platform was created in 2017. Its main objective is to establish the infrastructure to allow Spanish participation into international academic collaborative clinical trials, observational studies, and registries in pediatric oncology. The aim of this manuscript is to describe the activity conducted by ECLIM-SEHOP since its creation.
Methods: The platform's database was queried to provide an overview of the studies integrally and partially supported by the organization. Data on trial recruitment and set-up/conduct metrics since its creation until November 2023 were extracted.
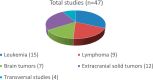
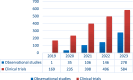
Results: ECLIM-SEHOP has supported 47 studies: 29 clinical trials and 18 observational studies/registries that have recruited a total of 5250 patients. Integral support has been given to 25 studies: 16 trials recruiting 584 patients and nine observational studies/registries recruiting 278 patients. The trials include front-line studies for leukemia, lymphoma, brain and solid extracranial tumors, and other key transversal topics such as off-label use of targeted therapies and survivorship. The mean time from regulatory authority submission to first patient recruited was 12.2 months and from first international site open to first Spanish site open was 31.3 months.
Discussion: ECLIM-SEHOP platform has remarkably improved the availability and accessibility of international academic clinical trials and has facilitated the centralization of resources in childhood cancer treatment. Despite the progressive improvement on clinical trial set-up metrics, timings should still be improved. The program has contributed to leveling survival rates in Spain with those of other European countries that presented major differences in the past.
Keywords: Clinical research; Clinical trials; Drug development; Metrics; Pediatric hematology and oncology.
© 2024. The Author(s).
Conflict of interest statement
A. Juan Ribelles has had a consulting/advisory role for Alexion and Bayer, has participated in educational activities organized by Eusa Pharma, Abbott, and Alexion, and has received travel expenses by Nestle and Alexion. F. Bautista has been a member of a data monitoring committee (DMC) in a clinical trial sponsored by Sanofi, had a consulting or advisory role for Bayer, Amgen, Roche Genentech, EusaPharma, and Eisai, and received honoraria from Servier for educational events (through his current affiliation). A. Cañete has had an advisory role for Norgine, Bayer, Roche, Eusa-Pharma, SERB, and participated in educational activities organized by Eusa Pharma and Norgine and received travel expenses from Eusa-pharma and Nestlé. A. Rubio-San Simon has had a advisory role for Eusa Pharma and Sanofi and has received travel expenses by Roche and Eusa Pharma. A. Alonso-Saladrigues (AAS): advisory boards (Novartis). S. Rives reports personal fees, honoraria from Novartis, Servier, Celgene/ Bristol Myers Squibb, Kite/ Gilead, and Amgen. Also reports being part of DMSB in clinical trial sponsored by Novartis and of a DMC in a clinical trial sponsored by Autolus. A. Lassaletta has had an advisory role for Jazz, Servier, and Alexion; has received travel expenses by Servier, and has participated in educational activities organized by Alexion. G. Ramírez-Villar has had an advisory role for Alexion, has received travel expenses by Nestlé and has participated in educational activities organized by Alexion. J.L.Fuster was a consultant/advisory member for Amgen, Jazz Pharmaceuticals, and Novartis, received honoraria from Amgen, Servier, Jazz Pharmaceuticals, Pfizer, and Novartis for speaking at symposia and support from Servier and Jazz Pharmaceuticals for attending symposia. C. Diaz-de-Heredia is a member of study steering committees for clinical trials sponsored by Novartis, had a consulting or advisory role for Novartis, Jazz Pharmaceuticals, Biotest and Vertex, has participated in educational activities organized by Novartis and Jazz Pharmaceuticals and received travel expenses from Jazz Pharmaceuticals and Novartis. J. Verdú-Amorós has received honoraria by Servier and received travel expenses by Servier and EusaPharma. A. Molinés has participated in educational activities organized by Servier receiving travel and accommodation expenses. L. Moreno has been member of data monitoring committees for clinical trials sponsored by Novartis, Actuate Therapeutics, Shionogi and Incyte; had a consulting role for Novartis, Norgine, Boehringer, Ymabs, Bayer, Abbvie, BMS and Shionogi; participated in educational activities organized by Bayer and Eusa Pharma and received travel expenses from Eusa Pharma. He is President Elect of SIOPEN (European neuroblastoma research cooperative group), an organization which receives royalties for the sales of dinutuximab beta. A. Fernández-Teijeiro has had an advisory role for AstraZeneca, SOBI, Takeda, Norgine, Clinigen, Bayer, Roche, MERCK SHARP & DOHME, Novartis and Takeda; participated in educational activities organized by Amgen, Eusa Pharma, Servier and Takeda and received travel expenses from Servier, Amgen and Gilead. The other authors declare no conflict of interest.
Figures
References
-
- Cañete Nieto A, Pardo Romaguera E, Muñoz López A, Valero Poveda S, Porta Cebolla S, Barreda Reines MS, et al. Cáncer infantil en España. Estadísticas 1980–2021. Registro Español de Tumores Infantiles (RETI-SEHOP). Valencia: Universitat de València; 2022.
-
- Peris-Bonet R. Incidencia y supervivencia del cáncer infantil. In: Madero L, Lassaleta A, Sevilla J, editors. Hematol y Oncol Pediátricas. 3rd ed. Madrid: Ergon; 2015. p. 263–70.
-
- Cañete Nieto A, Pardo Romaguera E, Alfonso Comos P, Valero Poveda S, Fernández Férriz A, Porta Cebolla S, et al. Cáncer infantil en España. Estadísticas 1980–2022. Registro Español de Tumores Infantiles (RETI-SEHOP). Valencia: Universitat de València (Edición preliminar); 2023.
-
- Botta L, Gatta G, Capocaccia R, Stiller C, Cañete A, Dal Maso L, et al. Long-term survival and cure fraction estimates for childhood cancer in Europe (EUROCARE-6): results from a population-based study. Lancet Oncol. 2022;23(12):1525–36. 10.1016/S1470-2045(22)00637-4. 10.1016/S1470-2045(22)00637-4 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous