Necroptosis blockade prevents lung injury in severe influenza
- PMID: 38600381
- PMCID: PMC11151938
- DOI: 10.1038/s41586-024-07265-8
Necroptosis blockade prevents lung injury in severe influenza
Abstract
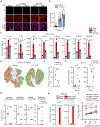
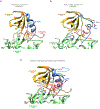
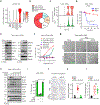
Severe influenza A virus (IAV) infections can result in hyper-inflammation, lung injury and acute respiratory distress syndrome1-5 (ARDS), for which there are no effective pharmacological therapies. Necroptosis is an attractive entry point for therapeutic intervention in ARDS and related inflammatory conditions because it drives pathogenic lung inflammation and lethality during severe IAV infection6-8 and can potentially be targeted by receptor interacting protein kinase 3 (RIPK3) inhibitors. Here we show that a newly developed RIPK3 inhibitor, UH15-38, potently and selectively blocked IAV-triggered necroptosis in alveolar epithelial cells in vivo. UH15-38 ameliorated lung inflammation and prevented mortality following infection with laboratory-adapted and pandemic strains of IAV, without compromising antiviral adaptive immune responses or impeding viral clearance. UH15-38 displayed robust therapeutic efficacy even when administered late in the course of infection, suggesting that RIPK3 blockade may provide clinical benefit in patients with IAV-driven ARDS and other hyper-inflammatory pathologies.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
S.N., G.D.C., A.D. and S.B. are listed as co-inventors on patent applications related to the UH15 series of compounds filed by Tufts University, the University of Houston, and the Institute for Cancer Research, Fox Chase Cancer Center. L.C.F., G.D.C., A.D. and S.B. hold equity in Vaayu Therapeutics. G.D.C. holds equity in Denali Therapeutics and has received royalties from Brigham & Women’s Hospital. The other authors declare no competing interests.
Figures





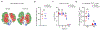




Comment in
-
RIPK3 inhibitor prevents lung damage in severe influenza infection.Nat Rev Drug Discov. 2024 Jun;23(6):417. doi: 10.1038/d41573-024-00072-w. Nat Rev Drug Discov. 2024. PMID: 38658639 No abstract available.
References
-
- Mauad T et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med 181, 72–79 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI144828/AI/NIAID NIH HHS/United States
- R56 AI168087/AI/NIAID NIH HHS/United States
- R01 AI135025/AI/NIAID NIH HHS/United States
- 75N93021C00016/AI/NIAID NIH HHS/United States
- R01 AI144400/AI/NIAID NIH HHS/United States
- R01 HL170121/HL/NHLBI NIH HHS/United States
- R35 CA231620/CA/NCI NIH HHS/United States
- S10 OD030332/OD/NIH HHS/United States
- R01 AI171568/AI/NIAID NIH HHS/United States
- R21 AI164003/AI/NIAID NIH HHS/United States
- R21 AI168799/AI/NIAID NIH HHS/United States
- 75N93021C00018/AI/NIAID NIH HHS/United States
- R37 AI044828/AI/NIAID NIH HHS/United States
- R01 CA269975/CA/NCI NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
- 75N93019C00052/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

