An integrated toolkit for human microglia functional genomics
- PMID: 38600587
- PMCID: PMC11005142
- DOI: 10.1186/s13287-024-03700-9
An integrated toolkit for human microglia functional genomics
Abstract
Background: Microglia, the brain's resident immune cells, play vital roles in brain development, and disorders like Alzheimer's disease (AD). Human iPSC-derived microglia (iMG) provide a promising model to study these processes. However, existing iMG generation protocols face challenges, such as prolonged differentiation time, lack of detailed characterization, and limited gene function investigation via CRISPR-Cas9.
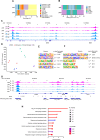
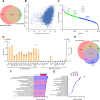
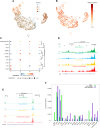
Methods: Our integrated toolkit for in-vitro microglia functional genomics optimizes iPSC differentiation into iMG through a streamlined two-step, 20-day process, producing iMG with a normal karyotype. We confirmed the iMG's authenticity and quality through single-cell RNA sequencing, chromatin accessibility profiles (ATAC-Seq), proteomics and functional tests. The toolkit also incorporates a drug-dependent CRISPR-ON/OFF system for temporally controlled gene expression. Further, we facilitate the use of multi-omic data by providing online searchable platform that compares new iMG profiles to human primary microglia: https://sherlab.shinyapps.io/IPSC-derived-Microglia/ .
Results: Our method generates iMG that closely align with human primary microglia in terms of transcriptomic, proteomic, and chromatin accessibility profiles. Functionally, these iMG exhibit Ca2 + transients, cytokine driven migration, immune responses to inflammatory signals, and active phagocytosis of CNS related substrates including synaptosomes, amyloid beta and myelin. Significantly, the toolkit facilitates repeated iMG harvesting, essential for large-scale experiments like CRISPR-Cas9 screens. The standalone ATAC-Seq profiles of our iMG closely resemble primary microglia, positioning them as ideal tools to study AD-associated single nucleotide variants (SNV) especially in the genome regulatory regions.
Conclusions: Our advanced two-step protocol rapidly and efficiently produces authentic iMG. With features like the CRISPR-ON/OFF system and a comprehensive multi-omic data platform, our toolkit equips researchers for robust microglial functional genomic studies. By facilitating detailed SNV investigation and offering a sustainable cell harvest mechanism, the toolkit heralds significant progress in neurodegenerative disease drug research and therapeutic advancement.
Keywords: CRISPR; Chromatin accessibility (ATAC-Seq); Functional genomics; Microglia; Neurodegenerative diseases; Proteomics; iPSC-derived microglia (iMG).
© 2024. The Author(s).
Conflict of interest statement
Authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

