Early spinal muscular atrophy treatment following newborn screening: A 20-month review of the first Italian regional experience
- PMID: 38600653
- PMCID: PMC11093231
- DOI: 10.1002/acn3.52018
Early spinal muscular atrophy treatment following newborn screening: A 20-month review of the first Italian regional experience
Abstract
Objectives: Mandatory newborn screening (NBS) for spinal muscular atrophy (SMA) was implemented for the first time in Italy at the end of 2021, allowing the identification and treatment of patients at an asymptomatic stage.
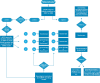
Methods: DNA samples extracted from dried blood spot (DBS) from newborns in Apulia region were analysed for SMA screening by using a real-time PCR-based assay. Infants harbouring homozygous deletion of SMN1 exon 7 confirmed by diagnostic molecular tests underwent clinical and neurophysiological assessment and received a timely treatment.
Results: Over the first 20 months since regional NBS introduction, four out of 42,492 (0.009%) screened children were found to carry a homozygous deletion in the exon 7 of SMN1 gene, with an annual incidence of 1:10,623. No false negatives were present. Median age at diagnosis was 7 days and median age at treatment was 20.5 days. Three of them had two copies of SMN2 and received gene therapy, while the one with three SMN2 copies was treated with nusinersen. All but one were asymptomatic at birth, showed no clinical signs of disease after a maximum follow-up of 16 months and reached motor milestones appropriate with their age. The minimum interval between diagnosis and the treatment initiation was 9 days.
Interpretation: The timely administration of disease-modifying therapies prevented presymptomatic subjects to develop disease symptoms. Mandatory NBS for SMA should be implemented on a national scale.
© 2024 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors declare no existing conflict of interests.
Figures

References
-
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile‐onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723‐1732. - PubMed
-
- Mendell JR, Al‐Zaidy S, Shell R, et al. Single‐dose gene‐replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713‐1722. - PubMed
-
- Baranello G, Darras BT, Day JW, et al. Risdiplam in type 1 spinal muscular atrophy. N Engl J Med. 2021;384(10):915‐923. - PubMed
-
- Office of the Commissioner . FDA Approves First Drug for Spinal Muscular Atrophy. FDA; 2020. https://www.fda.gov/news‐events/press‐announcements/fda‐approves‐first‐d...
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

