A Randomized Evaluation of MoodFX, a Patient-Centred e-Health Tool to Support Outcome Measurement for Depression: Une évaluation randomisée de MoodFX, un outil de santé en ligne centré sur le patient pour soutenir la mesure du résultat dans la dépression
- PMID: 38600892
- PMCID: PMC11168344
- DOI: 10.1177/07067437241245331
A Randomized Evaluation of MoodFX, a Patient-Centred e-Health Tool to Support Outcome Measurement for Depression: Une évaluation randomisée de MoodFX, un outil de santé en ligne centré sur le patient pour soutenir la mesure du résultat dans la dépression
Abstract
Background: e-Health tools using validated questionnaires to assess outcomes may facilitate measurement-based care for psychiatric disorders. MoodFX was created as a free online symptom tracker to support patients for outcome measurement in their depression treatment. We conducted a pilot randomized evaluation to examine its usability, and clinical utility.
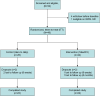
Methods: Patients presenting with a major depressive episode (within a major depressive or bipolar disorder) were randomly assigned to receive either MoodFX or a health information website as the intervention and control condition, respectively, with follow-up assessment surveys conducted online at baseline, 8 weeks and 6 months. The primary usability outcomes included the percentage of patients with self-reported use of MoodFX 3 or more times during follow up (indicating minimally adequate usage) and usability measures based on the System Usability Scale (SUS). Secondary clinical outcomes included the Quick Inventory of Depressive Symptomatology, Self-Rated (QIDS-SR) and Patient Health Questionnaire (PHQ-9).
Results: Forty-nine participants were randomized (24 to MoodFX and 25 to the control condition). Of the 23 participants randomized to MoodFX who completed the user survey, 18 (78%) used MoodFX 3 or more times over the 6 months of the study. The mean SUS score of 72.7 (65th-69th percentile) represents good usability. Compared to the control group, the MoodFX group had significantly better improvement on QIDS-SR and PHQ-9 scores, with large effect sizes and higher response rates at 6 months. There were no differences between conditions on other secondary outcomes such as functioning and quality of life.
Conclusion: MoodFX demonstrated good usability and was associated with reduction in depressive symptoms. This pilot study supports the use of digital tools in depression treatment.
Contexte: Les outils de santé en ligne qui utilisent des questionnaires validés pour évaluer les résultats peuvent faciliter les soins basés sur la mesure (SBM) pour les troubles psychiatriques. MoodFX a été créé à titre de suivi des symptômes en ligne gratuit pour soutenir la mesure des résultats des patients pour leur traitement de la dépression. Nous avons mené une évaluation randomisée pilote pour en examiner l’utilisabilité et l’utilité clinique.
Méthodes: Les patients présentant un épisode dépressif majeur (dans le cadre d’un trouble dépressif majeur ou d’un trouble bipolaire) ont été assignés au hasard à recevoir soit un MoodFX ou un site Web d’information de santé comme condition d’intervention et de contrôle, respectivement, avec des sondages d’évaluation de suivi menés en ligne à la base, après 8 semaines et 6 mois. Les premiers résultats d’utilisabilité comportaient le pourcentage de patients ayant un usage auto-déclaré de MoodFix trois fois ou plus durant le suivi (indiquant un usage minimalement adéquat) et des mesures d’utilisabilité basées sur l’Échelle d’utilisabilité du système (EUS). Les résultats cliniques secondaires comprenaient l’Inventaire rapide de la symptomatologie dépressive auto-déclarée (QIDS-SR) et le questionnaire de santé du patient (PHQ-9).
Résultats: Quarante-neuf participants ont été randomisés (24 au MoodFX et 25 à la condition de contrôle). Sur les 23 participants randomisés au MoodFX qui ont terminé le sondage de l’utilisateur, 18 (78%) ont utilisé MoodFX trois fois ou plus au cours des six mois de l’étude. Le score EUS moyen de 72,7 (65–69e percentile) représente une bonne utilisabilité. Comparé au groupe témoin, le groupe MoodFX avait une amélioration significativement meilleure aux scores QIDS-SR et PHQ-9 avec de grandes tailles d’effet et plus de taux de réponses à 6 mois. Il n’y avait pas de différences entre les conditions aux autres résultats secondaires comme le fonctionnement et la qualité de vie.
Conclusion: MoodFX a démontré une bonne utilisabilité et était associé à la réduction des symptômes dépressifs. Cette étude pilote soutient l’usage des outils numériques dans le traitement de la dépression.
Keywords: antidepressants; antidépresseurs; depressive disorders; e-mental health; essai randomisé contrôlé; randomized controlled trial; santé mentale en ligne; troubles dépressifs.
Plain language summary
E-health tools may be useful for measuring and tracking symptoms and other outcomes during treatment for depression. This study is a randomized evaluation of MoodFX, a free web-based app that helps patients track their symptoms using validated questionnaires, and also offers depression information and self-management tips. A total of 49 participants with clinical depression were randomized to using MoodFX or a health information website, for 6 months. In a survey, the participants that used MoodFX found it easy and useful to use. In addition, the participants that used MoodFX had greater improvement in depressive symptoms after 6 months, compared to those who used the health information website. These results suggest that MoodFX may be a useful tool to monitor outcomes and support depression treatment.
Conflict of interest statement
Declaration of Conflicting InterestsRWL has received honoraria for ad hoc speaking or advising/consulting, or received research funds, from: Abbvie, Asia-Pacific Economic Cooperation, Bausch, BC Leading Edge Foundation, Brain Canada, Canadian Institutes of Health Research, Canadian Network for Mood and Anxiety Treatments, CB Solutions, Carnot, Genome BC, Grand Challenges Canada, Healthy Minds Canada, Janssen, Lundbeck, Michael Smith Foundation for Health Research, MITACS, Neurotorium, Ontario Brain Institute, Otsuka, Pfizer/Viatris, Shanghai Mental Health Center, Unity Health, Vancouver Coastal Health Research Institute, and VGH-UBCH Foundation. AD was partly supported by an unrestricted fellowship grant from Janssen Canada and has received honoraria for ad hoc speaking or advising/consulting, or received research funds, from: AbbVie, Canadian Institutes of Health Research and Otsuka. JJN was partially supported by a Marshall/Institute of Mental Health Fellowship Award and received unrestricted research funding from the BC Cancer Foundation, with funds originating from Pfizer. KK is supported as a health professional investigator by Michael Smith Health Research Foundation of BC, Vancouver Coastal Health Research Institute and VGH & UBC Hospital Foundation and has previously served on the scientific advisory board of AbbVie. EEM has received support for patient education activities from Otsuka-Lundbeck. LNY has received honoraria for ad hoc speaking or advising/consulting, or received research funds, from: AbbVie, Alkermes, Allergan, Canadian Institutes of Health Research, Canadian Network for Mood and Anxiety Treatments, Dainippon Sumitomo Pharma, GlaxoSmithKline, Intracellular Therapies, Lundbeck, Merck, Otsuka, Sanofi, and Sunovion. VWL, VCE, JS, DKD, DDG, AA, EMT and JKM have no disclosures.
Figures

References
-
- Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx), https://vizhub.healthdata.org/gbd-results/ (2023, accessed 4 March 2023).
-
- Lam RW, Parikh SV, Michalak EE, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) consensus recommendations for functional outcomes in major depressive disorder. Ann Clin Psychiatry. 2015;27:142–149. - PubMed
-
- Herrman H, Kieling C, McGorry P, et al. Reducing the global burden of depression: a Lancet-World Psychiatric Association Commission. Lancet. 2019;393:e42–e43. - PubMed
-
- Zhu M, Hong RH, Yang T, et al. The efficacy of measurement-based care for depressive disorders: systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry. 2021;82:20210928. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

