Repetitive pulsed-wave ultrasound stimulation suppresses neural activity by modulating ambient GABA levels via effects on astrocytes
- PMID: 38601023
- PMCID: PMC11004293
- DOI: 10.3389/fncel.2024.1361242
Repetitive pulsed-wave ultrasound stimulation suppresses neural activity by modulating ambient GABA levels via effects on astrocytes
Abstract
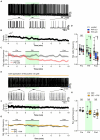
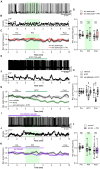
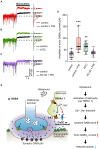
Ultrasound is highly biopermeable and can non-invasively penetrate deep into the brain. Stimulation with patterned low-intensity ultrasound can induce sustained inhibition of neural activity in humans and animals, with potential implications for research and therapeutics. Although mechanosensitive channels are involved, the cellular and molecular mechanisms underlying neuromodulation by ultrasound remain unknown. To investigate the mechanism of action of ultrasound stimulation, we studied the effects of two types of patterned ultrasound on synaptic transmission and neural network activity using whole-cell recordings in primary cultured hippocampal cells. Single-shot pulsed-wave (PW) or continuous-wave (CW) ultrasound had no effect on neural activity. By contrast, although repetitive CW stimulation also had no effect, repetitive PW stimulation persistently reduced spontaneous recurrent burst firing. This inhibitory effect was dependent on extrasynaptic-but not synaptic-GABAA receptors, and the effect was abolished under astrocyte-free conditions. Pharmacological activation of astrocytic TRPA1 channels mimicked the effects of ultrasound by increasing the tonic GABAA current induced by ambient GABA. Pharmacological blockade of TRPA1 channels abolished the inhibitory effect of ultrasound. These findings suggest that the repetitive PW low-intensity ultrasound used in our study does not have a direct effect on neural function but instead exerts its sustained neuromodulatory effect through modulation of ambient GABA levels via channels with characteristics of TRPA1, which is expressed in astrocytes.
Keywords: TRPA1; ambient GABA; astrocyte; network activity; ultrasound neuromodulation.
Copyright © 2024 Mishima, Komano, Tabaru, Kofuji, Saito, Ugawa and Terao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Cl⁻ homeodynamics in gap junction-coupled astrocytic networks on activation of GABAergic synapses.J Physiol. 2013 Aug 15;591(16):3901-17. doi: 10.1113/jphysiol.2013.257162. Epub 2013 Jun 3. J Physiol. 2013. PMID: 23732644 Free PMC article.
-
Tonic GABAA Conductance Favors Spike-Timing-Dependent over Theta-Burst-Induced Long-Term Potentiation in the Hippocampus.J Neurosci. 2020 May 27;40(22):4266-4276. doi: 10.1523/JNEUROSCI.2118-19.2020. Epub 2020 Apr 23. J Neurosci. 2020. PMID: 32327534 Free PMC article.
-
Ultrasonic Neuromodulation via Astrocytic TRPA1.Curr Biol. 2019 Oct 21;29(20):3386-3401.e8. doi: 10.1016/j.cub.2019.08.021. Epub 2019 Oct 3. Curr Biol. 2019. PMID: 31588000
-
Tonic GABA inhibition in hippocampal dentate granule cells: its regulation and function in temporal lobe epilepsies.Acta Physiol (Oxf). 2013 Nov;209(3):199-211. doi: 10.1111/apha.12148. Epub 2013 Aug 8. Acta Physiol (Oxf). 2013. PMID: 23865761 Review.
-
Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability.Psychopharmacology (Berl). 2013 Nov;230(2):151-88. doi: 10.1007/s00213-013-3276-5. Epub 2013 Sep 27. Psychopharmacology (Berl). 2013. PMID: 24071826 Free PMC article. Review.
Cited by
-
Non-invasive therapeutics for neurotrauma: a mechanistic overview.Front Neurol. 2025 May 14;16:1560777. doi: 10.3389/fneur.2025.1560777. eCollection 2025. Front Neurol. 2025. PMID: 40438568 Free PMC article. Review.
-
The future of transcranial ultrasound as a precision brain interface.PLoS Biol. 2024 Oct 29;22(10):e3002884. doi: 10.1371/journal.pbio.3002884. eCollection 2024 Oct. PLoS Biol. 2024. PMID: 39471185 Free PMC article.
-
Piezo1 Mediates Ultrasound-Stimulated Dopaminergic Neuron Protection via Synaptic Vesicle Recycling and Ferroptosis Inhibition.Neurosci Bull. 2025 May 29. doi: 10.1007/s12264-025-01420-5. Online ahead of print. Neurosci Bull. 2025. PMID: 40439849
References
-
- Bosson A., Paumier A., Boisseau S., Jacquier-Sarlin M., Buisson A., Albrieux M. (2017). TRPA1 channels promote astrocytic Ca2+ hyperactivity and synaptic dysfunction mediated by oligomeric forms of amyloid-β peptide. Mol. Neurodegener. 12, 53–19. doi: 10.1186/s13024-017-0194-8, PMID: - DOI - PMC - PubMed
-
- Cadoni S., Demené C., Alcala I., Provansal M., Nguyen D., Nelidova D., et al. . (2023). Ectopic expression of a mechanosensitive channel confers spatiotemporal resolution to ultrasound stimulations of neurons for visual restoration. Nat. Nanotechnol. 18, 667–676. doi: 10.1038/s41565-023-01359-6, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

