Cannabidiol exerts multitarget immunomodulatory effects on PBMCs from individuals with psoriasis vulgaris
- PMID: 38601151
- PMCID: PMC11004238
- DOI: 10.3389/fimmu.2024.1373435
Cannabidiol exerts multitarget immunomodulatory effects on PBMCs from individuals with psoriasis vulgaris
Abstract
Introduction: The involvement of endocannabinoid system (ECS) in the inflammatory cascade, and the ability of phytocannabinoids, endocannabinoids and their synthetic analogues to modulate it has become an interesting research area for new therapeutic approaches in inflammatory skin diseases. Cannabidiol (CBD) appears to be the most promising among phytocannabinoids, due to the lack of psychotropic effects and low toxicity profile. Its anti-inflammatory action has been highlighted in different preclinical models, ranging from experimental colitis to arthritis and neuroinflammation. Our aim was to evaluate CBD immune-modulatory effects in peripheral blood mononuclear cells (PBMC) of psoriasis individuals with particular attention to both innate and adaptative immune arms.
Methods: We performed in vitro immune functional experiments to analyze CBD action on various immune cells active in psoriatic lesions.
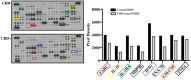
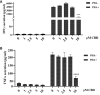
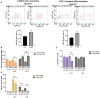
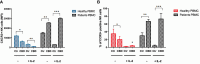
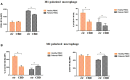
Results: The results showed that CBD produced a shift from Th1 to Th2 response, while boosting cytotoxic activity of Natural Killer (NK) cells. Furthermore, it also exerted a potent action on monocyte differentiation as, after CBD treatment, monocytes from psoriatic individuals were unable to migrate in response to inflammatory stimuli and to fully differentiate into mature dendritic cells. Finally, a M2 skewing of monocyte-derived macrophages by CBD also contributed to the fine tuning of the magnitude of immune responses.
Conclusions: These data uncover new potential immunomodulatory properties of this cannabinoid suggesting a possible therapeutic action in the treatment of multiple inflammatory skin diseases.
Keywords: CBD; dendritic cells; macrophage; monocyte; natural killer; psoriasis.
Copyright © 2024 Pagano, Ciaglia, Coppola, Lopardo, Raimondo, Giuseppe, Lembo, Laezza and Bifulco.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. (2013) 133:377–85. doi: 10.1038/jid.2012.339 - DOI - PubMed
-
- Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: Exploiting pathophysiological pathways for precision medicine. Clin Exp Rheumatol. (2015) 33:S2–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

