Novel immunotherapy combinations in neoadjuvant non-small cell lung cancer (NSCLC): a better chance at cure?
- PMID: 38601451
- PMCID: PMC11002516
- DOI: 10.21037/tlcr-23-735
Novel immunotherapy combinations in neoadjuvant non-small cell lung cancer (NSCLC): a better chance at cure?
Keywords: Neoadjuvant non-small cell lung cancer (neoadjuvant NSCLC); cancer immunotherapy; platform trial.
Conflict of interest statement
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-735/coif). A.I.S. is an inventor of patents related to cancer immunotherapy, some of which have been licensed to Lyell Immunopharma. M.D. has participated in advisory boards for Advarra, Astra Zeneca, Bristol Myer Squibb, Catalyst Pharmaceuticals, Gilead, Guardant, Janssen, Novocure, Regeneron, Genzyme and Sanofi; has provided consulting services for Eurofins, Abbvie, and Janssen; has received institutional grant funding from Merck, Genentech, CellSight, Novartis, Varian, and Verily; is the President of the Association of Northern California Oncologists; and has received travel funds and honoraria for speaking at various meetings related to cancer immunotherapy (Plexus, IDEO, Springer, Medical Educator Consortium, Dedham Group, DAVA Oncology, MJH Healthcare Holdings, ANCO, Aptitude Health, Med Learning Group, Curio, and Triptych Health). The authors have no other conflicts of interest to declare.
Figures
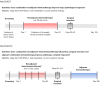
Comment on
-
Neoadjuvant Durvalumab Alone or Combined with Novel Immuno-Oncology Agents in Resectable Lung Cancer: The Phase II NeoCOAST Platform Trial.Cancer Discov. 2023 Nov 1;13(11):2394-2411. doi: 10.1158/2159-8290.CD-23-0436. Cancer Discov. 2023. PMID: 37707791 Free PMC article. Clinical Trial.
References
Publication types
LinkOut - more resources
Full Text Sources
