CD14 facilitates perinatal human cytomegalovirus infection in biliary epithelial cells via CD55
- PMID: 38601478
- PMCID: PMC11002872
- DOI: 10.1016/j.jhepr.2024.101018
CD14 facilitates perinatal human cytomegalovirus infection in biliary epithelial cells via CD55
Abstract
Background & aims: A high human cytomegalovirus (HCMV) infection rate accompanied by an increased level of bile duct damage is observed in the perinatal period. The possible mechanism was investigated.
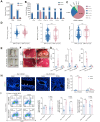
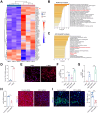
Methods: A total of 1,120 HCMV-positive and 9,297 HCMV-negative children were recruited, and depending on age, their liver biochemistry profile was compared. Fetal and infant biliary epithelial cells (F-BECs and I-BECs, respectively) were infected with HCMV, and the differences in cells were revealed by proteomic analysis. Protein-protein interactions were examined by coimmunoprecipitation and mass spectrometry analyses. A murine cytomegalovirus (MCMV) infection model was established to assess treatment effects.
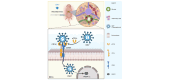
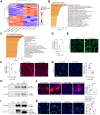
Results: Perinatal HCMV infection significantly increased the level of bile duct damage. Neonatal BALB/c mice inoculated with MCMV showed obvious inflammation in the portal area with an abnormal bile duct structure. Proteomics analysis showed higher CD14 expression in F-BECs than in I-BECs. CD14 siRNA administration hindered HCMV infection, and CD14-knockout mice showed lower MCMV-induced bile duct damage. HCMV infection upregulated CD55 and poly ADP-ribose polymerase-1 (PARP-1) expression in F-BECs. Coimmunoprecipitation and mass spectrometry analyses revealed formation of the CD14-CD55 complex. siRNA-mediated inhibition of CD55 expression reduced sCD14-promoted HCMV replication in F-BECs. In MCMV-infected mice, anti-mouse CD14 antibody and PARP-1 inhibitor treatment diminished cell death, ameliorated bile duct damage, and reduced mortality.
Conclusions: CD14 facilitates perinatal HCMV infection in BECs via CD55, and PARP-1-mediated cell death was detected in perinatal cytomegalovirus-infected BECs. These results provide new insight into the treatment of perinatal HCMV infection with bile duct damage.
Impact and implications: Perinatal human cytomegalovirus (HCMV) infection is associated with bile duct damage, but the underlying mechanism is still unknown. We discovered that CD14 expression is increased in biliary epithelial cells during perinatal HCMV infection and facilitates viral entry through CD55. We also detected PARP-1-mediated cell death in perinatal HCMV-infected biliary epithelial cells. We showed that blocking CD14 or inhibiting PARP-1 reduced bile duct damage and mortality in a mouse model of murine cytomegalovirus infection. Our findings provide a new insight into therapeutic strategies for perinatal HCMV infection.
Keywords: Biliary epithelial cells; CD14; CD55; Cytomegalovirus; PARP-1; Perinatal period.
© 2024 The Authors.
Conflict of interest statement
The authors have declared that no conflict of interest exists. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



Similar articles
-
The perinatal infection of cytomegalovirus is an important etiology for biliary atresia in China.Clin Pediatr (Phila). 2012 Feb;51(2):109-13. doi: 10.1177/0009922811406264. Epub 2011 Dec 5. Clin Pediatr (Phila). 2012. PMID: 22144720
-
Role of PARP-1 in Human Cytomegalovirus Infection and Functional Partners Encoded by This Virus.Viruses. 2022 Sep 15;14(9):2049. doi: 10.3390/v14092049. Viruses. 2022. PMID: 36146855 Free PMC article.
-
Reactive oxygen species-induced parthanatos of immunocytes by human cytomegalovirus-associated substance.Microbiol Immunol. 2018 Apr;62(4):229-242. doi: 10.1111/1348-0421.12575. Epub 2018 Mar 2. Microbiol Immunol. 2018. PMID: 29350405 Free PMC article.
-
Establishment of a novel mouse model of ulcerative colitis with concomitant cytomegalovirus infection: in vivo identification of cytomegalovirus persistent infected cells.Inflamm Bowel Dis. 2013 Aug;19(9):1951-63. doi: 10.1097/MIB.0b013e318293c5bf. Inflamm Bowel Dis. 2013. PMID: 23839229
-
Modulation of the microenvironment by senescent biliary epithelial cells may be involved in the pathogenesis of primary biliary cirrhosis.J Hepatol. 2010 Aug;53(2):318-25. doi: 10.1016/j.jhep.2010.03.008. Epub 2010 May 5. J Hepatol. 2010. PMID: 20570384
References
-
- Zuhair M., Smit G.S.A., Wallis G., et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019;29 - PubMed
-
- Wood A.M., Hughes B.L. Detection and prevention of perinatal infection: cytomegalovirus and Zika virus. Clin Perinatol. 2018;45:307–323. - PubMed
-
- Coclite E., Di Natale C., Nigro G. Congenital and perinatal cytomegalovirus lung infection. J Matern Fetal Neonatal Med. 2013;26:1671–1675. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

