Study of the cellular and humoral immune responses to SARS-CoV-2 vaccination
- PMID: 38601689
- PMCID: PMC11004869
- DOI: 10.1016/j.heliyon.2024.e29116
Study of the cellular and humoral immune responses to SARS-CoV-2 vaccination
Abstract
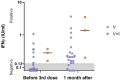
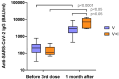
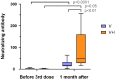
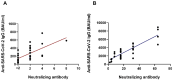
Our understanding of cellular immunity in response to COVID-19 infection or vaccination is limited because of less commonly used techniques. We investigated both the cellular and humoral immune responses before and after the administration of a third dose of the SARS-CoV-2 vaccine among a group of healthcare workers. Cellular immunity was evaluated using the VIDAS interferon-gamma (IFNγ) RUO test, which enables automated measurement of IFNγ levels after stimulating peripheral blood lymphocytes. Booster doses significantly enhanced both cellular and humoral immunity. Concerning cellular response, the booster dose increased the percentage of positive IFNγ release assay (IGRA) results but no difference in IFNγ release was found. The cellular response was not associated with protection against SARS-CoV-2 infection. Interestingly, vaccinated and infected healthcare workers exhibited the highest levels of anti-spike and neutralizing antibodies. In conclusion, the IGRA is a simple method for measuring cellular immune responses after vaccination. However, its usefulness as a complement to the study of humoral responses is yet to be demonstrated in future research.
Keywords: COVID-19 vaccination; Cellular immunity; Humoral immunity; Interferon gamma release assay; SARS-CoV-2.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Cellular and humoral immune response to SARS-CoV-2 vaccination and booster dose in immunosuppressed patients: An observational cohort study.J Clin Virol. 2022 Aug;153:105217. doi: 10.1016/j.jcv.2022.105217. Epub 2022 Jun 11. J Clin Virol. 2022. PMID: 35714462 Free PMC article.
-
Cell immunity to SARS-CoV-2 after natural infection and/or different vaccination regimens.Front Cell Infect Microbiol. 2024 Mar 20;14:1370859. doi: 10.3389/fcimb.2024.1370859. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38572317 Free PMC article.
-
Assessment of humoral and cellular immunity after bivalent BNT162b2 vaccination and potential association with reactogenicity.Clin Chem Lab Med. 2023 Feb 2;61(7):1343-1348. doi: 10.1515/cclm-2023-0055. Print 2023 Jun 27. Clin Chem Lab Med. 2023. PMID: 36722026
-
Boosting with Multiple Doses of mRNA Vaccine after Priming with Two Doses of Protein Subunit Vaccine MVC-COV1901 Elicited Robust Humoral and Cellular Immune Responses against Emerging SARS-CoV-2 Variants.Microbiol Spectr. 2022 Oct 26;10(5):e0060922. doi: 10.1128/spectrum.00609-22. Epub 2022 Aug 25. Microbiol Spectr. 2022. PMID: 36005765 Free PMC article.
-
T-Cell Mediated Response after Primary and Booster SARS-CoV-2 Messenger RNA Vaccination in Nursing Home Residents.J Am Med Dir Assoc. 2023 Feb;24(2):140-147.e2. doi: 10.1016/j.jamda.2022.11.024. Epub 2022 Dec 7. J Am Med Dir Assoc. 2023. PMID: 36587928 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

