Two-sample Mendelian randomization analysis of 91 circulating inflammatory protein levels and amyotrophic lateral sclerosis
- PMID: 38601850
- PMCID: PMC11004327
- DOI: 10.3389/fnagi.2024.1367106
Two-sample Mendelian randomization analysis of 91 circulating inflammatory protein levels and amyotrophic lateral sclerosis
Abstract
Introduction: Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease with poorly understood pathophysiology. Recent studies have highlighted systemic inflammation, especially the role of circulating inflammatory proteins, in ALS.
Methods: This study investigates the potential causal link between these proteins and ALS. We employed a two-sample Mendelian Randomization(MR) approach, analyzing data from large-scale genome-wide association studies to explore the relationship between 91 circulating inflammatory proteins and ALS. This included various MR methods like MR Egger, weighted median, and inverse-variance weighted, complemented by sensitivity analyses for robust results.
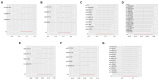
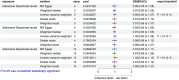
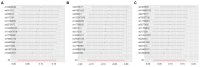
Results: Significant associations were observed between levels of inflammatory proteins, including Adenosine Deaminase, Interleukin-17C, Oncostatin-M, Leukemia Inhibitory Factor Receptor, and Osteoprotegerin, and ALS risk. Consistencies were noted across different P-value thresholds. Bidirectional MR suggested that ALS risk might influence levels of certain inflammatory proteins.
Discussion: Our findings, via MR analysis, indicate a potential causal relationship between circulating inflammatory proteins and ALS. This sheds new light on ALS pathophysiology and suggests possible therapeutic targets. Further research is required to confirm these results and understand the specific roles of these proteins in ALS.
Keywords: amyotrophic lateral sclerosis; circulating inflammatory protein; osteoprotegerin; tumor necrosis factor; two-sample mendelian randomization.
Copyright © 2024 Xiao, Gu, Feng and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Associations of the circulating levels of cytokines with risk of amyotrophic lateral sclerosis: a Mendelian randomization study.BMC Med. 2023 Feb 3;21(1):39. doi: 10.1186/s12916-023-02736-7. BMC Med. 2023. PMID: 36737740 Free PMC article.
-
Genome-wide association analysis reveals potential genetic correlation and causality between circulating inflammatory proteins and amyotrophic lateral sclerosis.Aging (Albany NY). 2024 May 30;16(11):9470-9484. doi: 10.18632/aging.205878. Epub 2024 May 30. Aging (Albany NY). 2024. PMID: 38819224 Free PMC article.
-
The causal association between epilepsy and amyotrophic lateral sclerosis: A two-sample Mendelian randomization study.Brain Behav. 2024 Oct;14(10):e70018. doi: 10.1002/brb3.70018. Brain Behav. 2024. PMID: 39328012 Free PMC article.
-
Bidirectional Mendelian randomization to explore the causal relationships between Sleep traits, Parkinson's disease and Amyotrophic lateral sclerosis.Sleep Med. 2022 Aug;96:42-49. doi: 10.1016/j.sleep.2022.03.024. Epub 2022 Apr 19. Sleep Med. 2022. PMID: 35594779
-
Interleukin-1 receptor antagonist, interleukin-2 receptor alpha subunit and amyotrophic lateral sclerosis.Eur J Neurol. 2020 Oct;27(10):1913-1917. doi: 10.1111/ene.14338. Epub 2020 Jun 8. Eur J Neurol. 2020. PMID: 32441415 Review.
Cited by
-
Elevated peripheral inflammation is associated with choroid plexus enlargement in independent sporadic amyotrophic lateral sclerosis cohorts.Fluids Barriers CNS. 2024 Oct 21;21(1):83. doi: 10.1186/s12987-024-00586-w. Fluids Barriers CNS. 2024. PMID: 39434103 Free PMC article.
-
Association between circulating inflammatory proteins and gout: A Mendelian randomization study.Medicine (Baltimore). 2025 May 16;104(20):e42379. doi: 10.1097/MD.0000000000042379. Medicine (Baltimore). 2025. PMID: 40388724 Free PMC article.
References
-
- Allen S. P., Hall B., Castelli L., Francis L., Woof R., Higginbottom A., et al. . (2018). Inosine reverses motor neuron toxicity observed in amyotrophic lateral sclerosis patient astrocytes with an adenosine deaminase deficiency. Biochimica et Biophysica Acta 1859:e23. doi: 10.1016/J.BBABIO.2018.09.071 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

