Bosutinib Stimulates Macrophage Survival, Phagocytosis, and Intracellular Killing of Bacteria
- PMID: 38602352
- PMCID: PMC11091880
- DOI: 10.1021/acsinfecdis.4c00086
Bosutinib Stimulates Macrophage Survival, Phagocytosis, and Intracellular Killing of Bacteria
Abstract
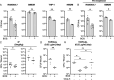
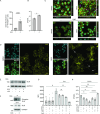
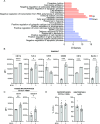
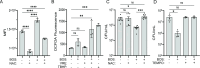
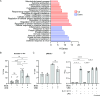
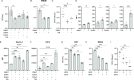
Host-acting compounds are emerging as potential alternatives to combating antibiotic resistance. Here, we show that bosutinib, an FDA-approved chemotherapeutic for treating chronic myelogenous leukemia, does not possess any antibiotic activity but enhances macrophage responses to bacterial infection. In vitro, bosutinib stimulates murine and human macrophages to kill bacteria more effectively. In a murine wound infection with vancomycin-resistant Enterococcus faecalis, a single intraperitoneal bosutinib injection or multiple topical applications on the wound reduce the bacterial load by approximately 10-fold, which is abolished by macrophage depletion. Mechanistically, bosutinib stimulates macrophage phagocytosis of bacteria by upregulating surface expression of bacterial uptake markers Dectin-1 and CD14 and promoting actin remodeling. Bosutinib also stimulates bacterial killing by elevating the intracellular levels of reactive oxygen species. Moreover, bosutinib drives NF-κB activation, which protects infected macrophages from dying. Other Src kinase inhibitors such as DMAT and tirbanibulin also upregulate expression of bacterial uptake markers in macrophages and enhance intracellular bacterial killing. Finally, cotreatment with bosutinib and mitoxantrone, another chemotherapeutic in clinical use, results in an additive effect on bacterial clearance in vitro and in vivo. These results show that bosutinib stimulates macrophage clearance of bacterial infections through multiple mechanisms and could be used to boost the host innate immunity to combat drug-resistant bacterial infections.
Keywords: Src kinases targeting; antibiotic resistance; immunomodulation; macrophages; phagocytosis; wound infection.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- da Silva R. A. G.; Wong J. J.; Antypas H.; Choo P. Y.; Goh K.; Jolly S.; Liang C.; Tay Kwan Sing L.; Veleba M.; Hu G.; Chen J.; Kline K. A. Mitoxantrone targets both host and bacteria to overcome vancomycin resistance in Enterococcus faecalis. Sci. Adv. 2023, 9 (8), eadd9280.10.1126/sciadv.add9280. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

