Difficult-to-treat (DTR) Pseudomonas aeruginosa harboring Verona-Integron metallo-β-lactamase (blaVIM): infection management and molecular analysis
- PMID: 38602418
- PMCID: PMC11064525
- DOI: 10.1128/aac.01474-23
Difficult-to-treat (DTR) Pseudomonas aeruginosa harboring Verona-Integron metallo-β-lactamase (blaVIM): infection management and molecular analysis
Abstract
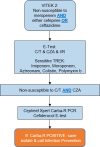
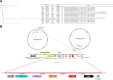
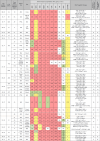
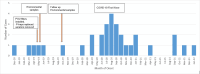
Pseudomonas aeruginosa harboring Verona Integron-encoded metallo-β-lactamase enzymes (VIM-CRPA) have been associated with infection outbreaks in several parts of the world. In the US, however, VIM-CRPA remain rare. Starting in December 2018, we identified a cluster of cases in our institution. Herein, we present our epidemiological investigation and strategies to control/manage these challenging infections. This study was conducted in a large academic healthcare system in Miami, FL, between December 2018 and January 2022. Patients were prospectively identified via rapid molecular diagnostics when cultures revealed carbapenem-resistant P. aeruginosa. Alerts were received in real time by the antimicrobial stewardship program and infection prevention teams. Upon alert recognition, a series of interventions were performed as a coordinated effort. A retrospective chart review was conducted to collect patient demographics, antimicrobial therapy, and clinical outcomes. Thirty-nine VIM-CRPA isolates led to infection in 21 patients. The majority were male (76.2%); the median age was 52 years. The majority were mechanically ventilated (n = 15/21; 71.4%); 47.6% (n = 10/21) received renal replacement therapy at the time of index culture. Respiratory (n = 20/39; 51.3%) or bloodstream (n = 13/39; 33.3%) were the most common sources. Most infections (n = 23/37; 62.2%) were treated with an aztreonam-avibactam regimen. Six patients (28.6%) expired within 30 days of index VIM-CRPA infection. Fourteen isolates were selected for whole genome sequencing. Most of them belonged to ST111 (12/14), and they all carried blaVIM-2 chromosomally. This report describes the clinical experience treating serious VIM-CRPA infections with either aztreonam-ceftazidime/avibactam or cefiderocol in combination with other agents. The importance of implementing infection prevention strategies to curb VIM-CRPA outbreaks is also demonstrated.
Keywords: Pseudomonas aeruginosa; Verona Integron metallo-beta-lactamase; antimicrobial stewardship; carbapenemase; infection prevention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Araos R, D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species, p 2686–2699. In Mandell, Douglass and Bennett’s principles and practice of infectious diseases
-
- COVID-19: U.S. impact on antimicrobial resistance, special report 2022. 2022. National Center for Emerging and Zoonotic Infectious Diseases
-
- Catho G, Martischang R, Boroli F, Chraïti MN, Martin Y, Koyluk Tomsuk Z, Renzi G, Schrenzel J, Pugin J, Nordmann P, Blanc DS, Harbarth S. 2021. Outbreak of Pseudomonas aeruginosa producing VIM carbapenemase in an intensive care unit and its termination by implementation of waterless patient care. Crit Care 25:301. doi:10.1186/s13054-021-03726-y - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

