The Effects of Pantoprazole on Kidney Outcomes: Post Hoc Observational Analysis from the COMPASS Trial
- PMID: 38602780
- PMCID: PMC11230723
- DOI: 10.1681/ASN.0000000000000356
The Effects of Pantoprazole on Kidney Outcomes: Post Hoc Observational Analysis from the COMPASS Trial
Abstract
Key Points:
In this post hoc analysis of a randomized controlled trial, the proton pump inhibitor pantoprazole led to a faster rate of eGFR decline as compared with placebo.
Additional studies are needed to determine the effect of proton pump inhibitors on those at higher risk of adverse kidney outcomes.
Background: Observational studies have found an association between proton pump inhibitor use and worsening kidney function. It is unclear whether these associations are causal. We conducted post hoc analyses to determine the effect of pantoprazole on kidney function using data from the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial, a 17,598-participant randomized trial comparing pantoprazole (8791) with placebo (8807).
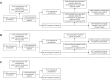
Methods: The primary outcome was the rate of eGFR change. Rate of eGFR change was based on the two eGFR measures available: the eGFR at randomization and at the open-label extension study that enrolled at trial conclusion. Secondary outcomes included incident CKD (defined by eGFR <60 ml/min per 1.73 m2 at open-label extension or case report forms) as well as AKI, acute nephritis, and nephrotic syndrome.
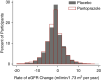
Results: Eight thousand nine hundred ninety-one of the 17,598 participants randomized to pantoprazole/placebo (51%) had eGFR recorded at baseline and open-label extension enrollment and were included in the rate of eGFR change population (mean age 67 [SD 8] years, 22% female, mean baseline eGFR 75 [SD 17.5] ml/min per 1.73 m2). The mean duration between randomization and open-label extension eGFR was 3.3 (SD 0.8) years. The placebo rate of eGFR change was −1.41 (SD 4.45) ml/min per 1.73 m2 per year. The pantoprazole rate of eGFR change was −1.64 (SD 4.47) ml/min per 1.73 m2 per year. In adjusted analyses, pantoprazole had a 0.27 ml/min per 1.73 m2 per year greater decline in eGFR (95% confidence interval [CI], 0.11 to 0.43). The odds ratio for the effect of pantoprazole on incident CKD was 1.11 (95% CI, 0.98 to 1.25) and on AKI was 0.89 (95% CI, 0.65 to 1.21). There were five nephrotic syndrome outcomes recorded and one event of acute nephritis.
Conclusions: In this post hoc analysis of the COMPASS trial, pantoprazole resulted in a statistically significant greater rate of eGFR decline as compared with placebo.
Clinical Trial registry name and registration number::
Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease (COMPASS),
Conflict of interest statement
Disclosure forms, as provided by each author, are available with the online version of the article at
Figures

References
-
- Rotermann M, Sanmartin C, Hennessy D, Arthur M. Prescription medication use by Canadians aged 6 to 79. Health Rep. 2014;25(6):3–9. PMID: 24941315 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

