Efficient Vascular and Neural Engraftment of Stem Cell-Derived Islets
- PMID: 38603470
- PMCID: PMC11189832
- DOI: 10.2337/db23-0123
Efficient Vascular and Neural Engraftment of Stem Cell-Derived Islets
Abstract
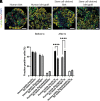
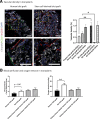
Pluripotent stem cell-derived islets (SC-islets) have emerged as a new source for β-cell replacement therapy. The function of human islet transplants is hampered by excessive cell death posttransplantation; contributing factors include inflammatory reactions, insufficient revascularization, and islet amyloid formation. However, there is a gap in knowledge of the engraftment process of SC-islets. In this experimental study, we investigated the engraftment capability of SC-islets at 3 months posttransplantation and observed that cell apoptosis rates were lower but vascular density was similar in SC-islets compared with human islets. Whereas the human islet transplant vascular structures were a mixture of remnant donor endothelium and ingrowing blood vessels, the SC-islets contained ingrowing blood vessels only. Oxygenation in the SC-islet grafts was twice as high as that in the corresponding grafts of human islets, suggesting better vascular functionality. Similar to the blood vessel ingrowth, reinnervation of the SC-islets was four- to fivefold higher than that of the human islets. Both SC-islets and human islets contained amyloid at 1 and 3 months posttransplantation. We conclude that the vascular and neural engraftment of SC-islets are superior to those of human islets, but grafts of both origins develop amyloid, with potential long-term consequences.
© 2024 by the American Diabetes Association.
Conflict of interest statement
Figures





References
-
- Maffi P, Secchi A. Clinical results of islet transplantation. Pharmacol Res 2015;98:86–91 - PubMed
-
- Eriksson O, Eich T, Sundin A, et al. . Positron emission tomography in clinical islet transplantation. Am J Transplant 2009;9:2816–2824 - PubMed
-
- Moberg L, Johansson H, Lukinius A, et al. . Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet 2002;360:2039–2045 - PubMed
-
- Lee Y, Ravazzola M, Park BH, Bashmakov YK, Orci L, Unger RH. Metabolic mechanisms of failure of intraportally transplanted pancreatic beta-cells in rats: role of lipotoxicity and prevention by leptin. Diabetes 2007;56:2295–2301 - PubMed
-
- Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes 2002;51:1362–1366 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

