Mechanisms underlying aryl hydrocarbon receptor-driven divergent macrophage function
- PMID: 38603630
- PMCID: PMC11199922
- DOI: 10.1093/toxsci/kfae050
Mechanisms underlying aryl hydrocarbon receptor-driven divergent macrophage function
Abstract
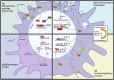
Macrophages play an essential role in the innate immune system by differentiating into functionally diverse subsets in order to fight infection, repair damaged tissues, and regulate inappropriate immune responses. This functional diversity stems from their ability to adapt and respond to signals in the environment, which is in part mediated through aryl hydrocarbon receptor (AHR)-signaling. AHR, an environmental sensor, can be activated by various ligands, ranging from environmental contaminants to microbially derived tryptophan metabolites. This review discusses what is currently known about how AHR-signaling influences macrophage differentiation, polarization, and function. By discussing studies that are both consistent and divergent, our goal is to highlight the need for future research on the mechanisms by which AHR acts as an immunological switch in macrophages. Ultimately, understanding the contexts in which AHR-signaling promotes and/or inhibits differentiation, proinflammatory functions, and immunoregulatory functions, will help uncover functional predictions of immunotoxicity following exposure to environmental chemicals as well as better design AHR-targeted immunotherapies.
Keywords: aryl hydrocarbon receptor; immunomodulation; immunosuppression; inflammation; macrophages.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Effects of an aryl hydrocarbon receptor ligand on human trophoblast cell development.Hum Reprod. 2025 Jun 1;40(6):1163-1172. doi: 10.1093/humrep/deaf075. Hum Reprod. 2025. PMID: 40294436
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Melatonin and Its Metabolites Can Serve as Agonists on the Aryl Hydrocarbon Receptor and Peroxisome Proliferator-Activated Receptor Gamma.Int J Mol Sci. 2023 Oct 23;24(20):15496. doi: 10.3390/ijms242015496. Int J Mol Sci. 2023. PMID: 37895177 Free PMC article.
-
Unraveling the significance of innate inflammation in vascular disease.Int Rev Immunol. 2025;44(5):229-244. doi: 10.1080/08830185.2025.2489346. Epub 2025 Apr 21. Int Rev Immunol. 2025. PMID: 40255209 Review.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
Cited by
-
Vertical Transfer of Maternal Gut Microbes to Offspring of Western Diet-Fed Dams Drives Reduced Levels of Tryptophan Metabolites and Postnatal Innate Immune Response.Nutrients. 2024 Jun 8;16(12):1808. doi: 10.3390/nu16121808. Nutrients. 2024. PMID: 38931163 Free PMC article.
-
Vascular inflammation in chronic kidney disease: the role of uremic toxins in macrophage activation.Front Cardiovasc Med. 2025 Mar 25;12:1574489. doi: 10.3389/fcvm.2025.1574489. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40201789 Free PMC article. Review.
-
Depletion of intrinsic renal macrophages with moderate-to-high expression of CD163, MRC1, PTH2R, PDE4D, and CUBN in regulating podocyte injury in diabetic nephropathy: a single-cell RNA sequencing analysis.Transl Androl Urol. 2024 Nov 30;13(11):2527-2552. doi: 10.21037/tau-24-569. Epub 2024 Nov 28. Transl Androl Urol. 2024. PMID: 39698573 Free PMC article.
-
Harnessing aryl hydrocarbon receptor dynamics: Unveiling therapeutic pathways in esophageal squamous cell carcinoma.World J Exp Med. 2024 Dec 20;14(4):98599. doi: 10.5493/wjem.v14.i4.98599. eCollection 2024 Dec 20. World J Exp Med. 2024. PMID: 39713081 Free PMC article.
-
Eucalyptus Wood Smoke Extract Elicits a Dose-Dependent Effect in Brain Endothelial Cells.Int J Mol Sci. 2024 Sep 24;25(19):10288. doi: 10.3390/ijms251910288. Int J Mol Sci. 2024. PMID: 39408618 Free PMC article.
References
-
- Alivernini S., MacDonald L., Elmesmari A., Finlay S., Tolusso B., Gigante M. R., Petricca L., Di Mario C., Bui L., Perniola S., et al. (2020). Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat. Med. 26, 1295–1306. 10.1038/s41591-020-0939-8 - DOI - PubMed
-
- Brebner K., Anisman H. (2000). Synergistic effects of interleukin-1NL, interleukin-6, and tumor necrosis factor-␣:central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology 22, 566–580. - PubMed

