A gain-of-function mutation in zinc cluster transcription factor Rob1 drives Candida albicans adaptive growth in the cystic fibrosis lung environment
- PMID: 38603707
- PMCID: PMC11037546
- DOI: 10.1371/journal.ppat.1012154
A gain-of-function mutation in zinc cluster transcription factor Rob1 drives Candida albicans adaptive growth in the cystic fibrosis lung environment
Abstract
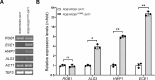
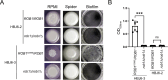
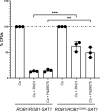
Candida albicans chronically colonizes the respiratory tract of patients with Cystic Fibrosis (CF). It competes with CF-associated pathogens (e.g. Pseudomonas aeruginosa) and contributes to disease severity. We hypothesize that C. albicans undergoes specific adaptation mechanisms that explain its persistence in the CF lung environment. To identify the underlying genetic and phenotypic determinants, we serially recovered 146 C. albicans clinical isolates over a period of 30 months from the sputum of 25 antifungal-naive CF patients. Multilocus sequence typing analyses revealed that most patients were individually colonized with genetically close strains, facilitating comparative analyses between serial isolates. We strikingly observed differential ability to filament and form monospecies and dual-species biofilms with P. aeruginosa among 18 serial isolates sharing the same diploid sequence type, recovered within one year from a pediatric patient. Whole genome sequencing revealed that their genomes were highly heterozygous and similar to each other, displaying a highly clonal subpopulation structure. Data mining identified 34 non-synonymous heterozygous SNPs in 19 open reading frames differentiating the hyperfilamentous and strong biofilm-former strains from the remaining isolates. Among these, we detected a glycine-to-glutamate substitution at position 299 (G299E) in the deduced amino acid sequence of the zinc cluster transcription factor ROB1 (ROB1G299E), encoding a major regulator of filamentous growth and biofilm formation. Introduction of the G299E heterozygous mutation in a co-isolated weak biofilm-former CF strain was sufficient to confer hyperfilamentous growth, increased expression of hyphal-specific genes, increased monospecies biofilm formation and increased survival in dual-species biofilms formed with P. aeruginosa, indicating that ROB1G299E is a gain-of-function mutation. Disruption of ROB1 in a hyperfilamentous isolate carrying the ROB1G299E allele abolished hyperfilamentation and biofilm formation. Our study links a single heterozygous mutation to the ability of C. albicans to better survive during the interaction with other CF-associated microbes and illuminates how adaptive traits emerge in microbial pathogens to persistently colonize and/or infect the CF-patient airways.
Copyright: © 2024 Gnaien et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

