An engineered in vitro model of the human myotendinous junction
- PMID: 38604466
- PMCID: PMC11088524
- DOI: 10.1016/j.actbio.2024.04.007
An engineered in vitro model of the human myotendinous junction
Abstract
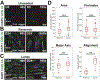
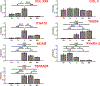
The myotendinous junction (MTJ) is a vulnerable region at the interface of skeletal muscle and tendon that forms an integrated mechanical unit. This study presents a technique for the spatially restrictive co-culture of human embryonic stem cell (hESC)-derived skeletal myocytes and primary tenocytes for two-dimensional modeling of the MTJ. Micropatterned lanes of extracellular matrix and a 2-well culture chamber define the initial regions of occupation. On day 1, both lines occupy less than 20 % of the initially vacant interstitial zone, referred to henceforth as the junction. Myocyte-tenocyte interdigitations are observed by day 7. Immunocytochemistry reveals enhanced organization and alignment of patterned myocyte and tenocyte features, as well as differential expression of multiple MTJ markers. On day 24, electrically stimulated junction myocytes demonstrate negative contractile strains, while positive tensile strains are exhibited by mechanically passive tenocytes at the junction. Unpatterned tenocytes distal to the junction experience significantly decreased strains in comparison to cells at the interface. Unpatterned myocytes have impaired organization and uncoordinated contractile behavior. These findings suggest that this platform is capable of inducing myocyte-tenocyte junction formation and mechanical coupling similar to the native MTJ, showing transduction of force across the cell-cell interface. STATEMENT OF SIGNIFICANCE: The myotendinous junction (MTJ) is an integrated structure that transduces force across the muscle-tendon boundary, making the region vulnerable to strain injury. Despite the clinical relevance, previous in vitro models of the MTJ lack the structure and mechanical accuracy of the native tissue and have difficulty transmitting force across the cell-cell interface. This study demonstrates an in vitro model of the MTJ, using spatially restrictive cues to inform human myocyte-tenocyte interactions and architecture. The model expressed MTJ markers and developed anisotropic myocyte-tenocyte integrations that resemble the native tissue and allow for force transduction from contracting myocytes to passive tenocyte regions. As such, this study presents a system capable of investigating development, injury, and pathology in the human MTJ.
Keywords: In vitro modeling; Micropattern; Myotendinous junction; Skeletal myocytes; Tenocytes.
Copyright © 2024 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro.Tissue Eng. 2006 Nov;12(11):3149-58. doi: 10.1089/ten.2006.12.3149. Tissue Eng. 2006. PMID: 17518629 Free PMC article.
-
Larger interface area at the human myotendinous junction in type 1 compared with type 2 muscle fibers.Scand J Med Sci Sports. 2023 Feb;33(2):136-145. doi: 10.1111/sms.14246. Epub 2022 Oct 28. Scand J Med Sci Sports. 2023. PMID: 36226768 Free PMC article.
-
3D Printing of a Biomimetic Myotendinous Junction Assisted by Artificial Intelligence.Biomater Sci. 2024 Nov 19;12(23):6047-6062. doi: 10.1039/d4bm00892h. Biomater Sci. 2024. PMID: 39446075
-
Engineering interfacial tissues: The myotendinous junction.APL Bioeng. 2024 Jun 3;8(2):021505. doi: 10.1063/5.0189221. eCollection 2024 Jun. APL Bioeng. 2024. PMID: 38841690 Free PMC article. Review.
-
Regeneration techniques for bone-to-tendon and muscle-to-tendon interfaces reconstruction.Br Med Bull. 2016 Mar;117(1):25-37. doi: 10.1093/bmb/ldv056. Epub 2016 Feb 2. Br Med Bull. 2016. PMID: 26837850 Review.
Cited by
-
hiPSC-derived cardiac fibroblasts dynamically enhance the mechanical function of hiPSC-derived cardiomyocytes on an engineered substrate.Front Bioeng Biotechnol. 2025 May 23;13:1546483. doi: 10.3389/fbioe.2025.1546483. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40486207 Free PMC article.
References
-
- Knudsen A, Mackey AL, Jakobsen JR, Krogsgaard MR, No demonstrable ultrastructural adaptation of the human myotendinous junction to immobilization or 4 weeks of heavy resistance training, Transl Sports Med. 4 (2021) 431–438. 10.1002/tsm2.243. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

