Chemical Strategies for the Detection and Elimination of Senescent Cells
- PMID: 38604701
- PMCID: PMC11079973
- DOI: 10.1021/acs.accounts.3c00794
Chemical Strategies for the Detection and Elimination of Senescent Cells
Abstract
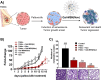
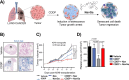
Cellular senescence can be defined as an irreversible stopping of cell proliferation that arises in response to various stress signals. Cellular senescence is involved in diverse physiological and pathological processes in different tissues, exerting effects on processes as differentiated as embryogenesis, tissue repair and remodeling, cancer, aging, and tissue fibrosis. In addition, the development of some pathologies, aging, cancer, and other age-related diseases has been related to senescent cell accumulation. Due to the complexity of the senescence phenotype, targeting senescent cells is not trivial, is challenging, and is especially relevant for in vivo detection in age-related diseases and tissue samples. Despite the elimination of senescent cells (senolysis) using specific drugs (senolytics) that have been shown to be effective in numerous preclinical disease models, the clinical translation is still limited due to the off-target effects of current senolytics and associated toxicities. Therefore, the development of new chemical strategies aimed at detecting and eliminating senescent cells for the prevention and selective treatment of senescence-associated diseases is of great interest. Such strategies not only will contribute to a deeper understanding of this rapidly evolving field but also will delineate and inspire new possibilities for future research.In this Account, we report our recent research in the development of new chemical approaches for the detection and elimination of senescent cells based on new probes, nanoparticles, and prodrugs. The designed systems take advantage of the over-representation in senescent cells of certain biomarkers such as β-galactosidase and lipofuscin. One- and two-photon probes, for higher tissue penetration, have been developed. Moreover, we also present a renal clearable fluorogenic probe for the in vivo detection of the β-galactosidase activity, allowing for correlation with the senescent burden in living animals. Moreover, as an alternative to molecular-based probes, we also developed nanoparticles for senescence detection. Besides, we describe advances in new therapeutic agents to selectively eradicate senescent cells using β-galactosidase activity-sensitive gated nanoparticles loaded with cytotoxic or senolytic agents or new prodrugs aiming to increase the selectivity and reduction of off-target toxicities of current drugs. Moreover, new advances therapies have been applied in vitro and in vivo. Studies with the probes, nanoparticles, and prodrugs have been applied in several in vitro and in vivo models of cancer, fibrosis, aging, and drug-induced cardiotoxicity in which senescence plays an important role. We discuss the benefits of these chemical strategies toward the development of more specific and sophisticated probes, nanoparticles, and prodrugs targeting senescent cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Lozano-Torres B.; Blandez J. F.; Galiana I.; García-Fernández A.; Alfonso M.; Marcos M. D.; Orzáez M.; Sancenón F.; Martínez-Máñez R. Real-Time In Vivo Detection of Cellular Senescence through the Controlled Release of the NIR Fluorescent Dye Nile Blue. Angew. Chem., Int. Ed. 2020, 59 (35), 15152–15156. 10.1002/anie.202004142. - DOI - PubMed
-
- Muñoz-Espín D.; Rovira M.; Galiana I.; Giménez C.; Lozano-Torres B.; Paez-Ribes M.; Llanos S.; Chaib S.; Muñoz-Martín M.; Ucero A. C.; Garaulet G.; Mulero F.; Dann S. G.; VanArsdale T.; Shields D. J.; Bernardos A.; Murguía J. R.; Martínez-Máñez R.; Serrano M. A Versatile Drug Delivery System Targeting Senescent Cells. EMBO Mol. Med. 2018, 10, e9355.10.15252/emmm.201809355. - DOI - PMC - PubMed
-
- González-Gualda E.; Pàez-Ribes M.; Lozano-Torres B.; Macias D.; Wilson J. R.; González-López C.; Ou H. L.; Mirón-Barroso S.; Zhang Z.; Lérida-Viso A.; Blandez J. F.; Bernardos A.; Sancenón F.; Rovira M.; Fruk L.; Martins C. P.; Serrano M.; Doherty G. J.; Martínez-Máñez R.; Muñoz-Espín D. Galacto-Conjugation of Navitoclax as an Efficient Strategy to Increase Senolytic Specificity and Reduce Platelet Toxicity. Aging Cell 2020, 19, e13142.10.1111/acel.13142. - DOI - PMC - PubMed

