Longitudinal analysis of PD-L1 expression in patients with relapsed NSCLC
- PMID: 38604811
- PMCID: PMC11015283
- DOI: 10.1136/jitc-2023-008592
Longitudinal analysis of PD-L1 expression in patients with relapsed NSCLC
Abstract
Background: The use and approval of immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) depends on PD-L1 expression in the tumor tissue. Nevertheless, PD-L1 often fails to predict response to treatment. One possible explanation could be a change in PD-L1 expression during the course of the disease and the neglect of reassessment. The purpose of this study was a longitudinal analysis of PD-L1 expression in patients with relapsed NSCLC.
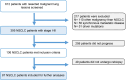
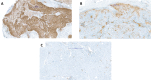
Methods: We retrospectively analyzed PD-L1 expression in patients with early-stage NSCLC and subsequent relapse in preoperative samples, matched surgical specimens and biopsy samples of disease recurrence. Ventana PD-L1 (SP263) immunohistochemistry assay was used for all samples. PD-L1 expression was scored based on clinically relevant groups (0%, 1%-49%, and ≥50%). The primary endpoint was the change in PD-L1 score group between preoperative samples, matched surgical specimens and relapsed tumor tissue.
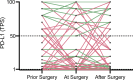
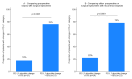
Results: 395 consecutive patients with stages I-III NSCLC and 136 (34%) patients with a subsequent relapse were identified. For 87 patients at least two specimens for comparison of PD-L1 expression between early stage and relapsed disease were available. In 72 cases, a longitudinal analysis between preoperative biopsy, the surgically resected specimen and biopsy of disease recurrence was feasible. When comparing preoperative and matched surgical specimens, a treatment-relevant conversion of PD-L1 expression group was found in 25 patients (34.7%). Neoadjuvant treatment showed no significant effect on PD-L1 alteration (p=0.39). In 32 (36.8%) out of 87 cases, a change in PD-L1 group was observed when biopsies of disease relapse were compared with early-stage disease. Adjuvant treatment was not significantly associated with a change in PD-L1 expression (p=0.53). 39 patients (54.2%) showed at least 1 change into a different PD-L1 score group during the course of disease. 14 patients (19.4%) changed the PD-L1 score group twice, 5 (6.9%) of them being found in all different score groups.
Conclusion: PD-L1 expression shows dynamic changes during the course of disease. There is an urgent need for consensus guidelines to define a PD-L1 testing strategy including time points of reassessment, the number of biopsies to be obtained and judgment of surgical specimens.
Keywords: Biomarker; Immune Checkpoint Inhibitor; Lung Cancer.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- U.S. Department of Health and . Lung and bronchus SEER 5-year relative survival rates, 2013-2019. SEER cancer Statistics. 2023. Available: https://seer.cancer.gov/statistics-network/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
