Reporting guidelines in medical artificial intelligence: a systematic review and meta-analysis
- PMID: 38605106
- PMCID: PMC11009315
- DOI: 10.1038/s43856-024-00492-0
Reporting guidelines in medical artificial intelligence: a systematic review and meta-analysis
Abstract
Background: The field of Artificial Intelligence (AI) holds transformative potential in medicine. However, the lack of universal reporting guidelines poses challenges in ensuring the validity and reproducibility of published research studies in this field.
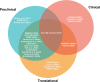
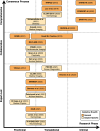
Methods: Based on a systematic review of academic publications and reporting standards demanded by both international consortia and regulatory stakeholders as well as leading journals in the fields of medicine and medical informatics, 26 reporting guidelines published between 2009 and 2023 were included in this analysis. Guidelines were stratified by breadth (general or specific to medical fields), underlying consensus quality, and target research phase (preclinical, translational, clinical) and subsequently analyzed regarding the overlap and variations in guideline items.
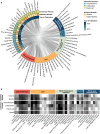
Results: AI reporting guidelines for medical research vary with respect to the quality of the underlying consensus process, breadth, and target research phase. Some guideline items such as reporting of study design and model performance recur across guidelines, whereas other items are specific to particular fields and research stages.
Conclusions: Our analysis highlights the importance of reporting guidelines in clinical AI research and underscores the need for common standards that address the identified variations and gaps in current guidelines. Overall, this comprehensive overview could help researchers and public stakeholders reinforce quality standards for increased reliability, reproducibility, clinical validity, and public trust in AI research in healthcare. This could facilitate the safe, effective, and ethical translation of AI methods into clinical applications that will ultimately improve patient outcomes.
Plain language summary
Artificial Intelligence (AI) refers to computer systems that can perform tasks that normally require human intelligence, like recognizing patterns or making decisions. AI has the potential to transform healthcare, but research on AI in medicine needs clear rules so caregivers and patients can trust it. This study reviews and compares 26 existing guidelines for reporting on AI in medicine. The key differences between these guidelines are their target areas (medicine in general or specific medical fields), the ways they were created, and the research stages they address. While some key items like describing the AI model recurred across guidelines, others were specific to the research area. The analysis shows gaps and variations in current guidelines. Overall, transparent reporting is important, so AI research is reliable, reproducible, trustworthy, and safe for patients. This systematic review of guidelines aims to increase the transparency of AI research, supporting an ethical and safe progression of AI from research into clinical practice.
© 2024. The Author(s).
Conflict of interest statement
D.T. holds shares in StratifAI GmbH and has received honoraria for lectures from Bayer. J.N.K. declares consulting services for Owkin, France, DoMore Diagnostics, Norway, Panakeia, UK, Scailyte, Switzerland, Cancilico, Germany, Mindpeak, Germany, MultiplexDx, Slovakia, and Histofy, UK; furthermore, he holds shares in StratifAI GmbH, Germany, has received a research grant by GSK, and has received honoraria by AstraZeneca, Bayer, Eisai, Janssen, MSD, BMS, Roche, Pfizer and Fresenius. All other authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

