Neurofeedback training of executive function in autism spectrum disorder: distinct effects on brain activity levels and compensatory connectivity changes
- PMID: 38605323
- PMCID: PMC11008042
- DOI: 10.1186/s11689-024-09531-2
Neurofeedback training of executive function in autism spectrum disorder: distinct effects on brain activity levels and compensatory connectivity changes
Abstract
Background: Deficits in executive function (EF) are consistently reported in autism spectrum disorders (ASD). Tailored cognitive training tools, such as neurofeedback, focused on executive function enhancement might have a significant impact on the daily life functioning of individuals with ASD. We report the first real-time fMRI neurofeedback (rt-fMRI NF) study targeting the left dorsolateral prefrontal cortex (DLPFC) in ASD.
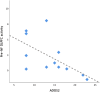
Methods: Thirteen individuals with autism without intellectual disability and seventeen neurotypical individuals completed a rt-fMRI working memory NF paradigm, consisting of subvocal backward recitation of self-generated numeric sequences. We performed a region-of-interest analysis of the DLPFC, whole-brain comparisons between groups and, DLPFC-based functional connectivity.
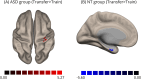
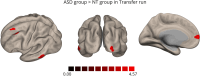
Results: The ASD and control groups were able to modulate DLPFC activity in 84% and 98% of the runs. Activity in the target region was persistently lower in the ASD group, particularly in runs without neurofeedback. Moreover, the ASD group showed lower activity in premotor/motor areas during pre-neurofeedback run than controls, but not in transfer runs, where it was seemingly balanced by higher connectivity between the DLPFC and the motor cortex. Group comparison in the transfer run also showed significant differences in DLPFC-based connectivity between groups, including higher connectivity with areas integrated into the multidemand network (MDN) and the visual cortex.
Conclusions: Neurofeedback seems to induce a higher between-group similarity of the whole-brain activity levels (including the target ROI) which might be promoted by changes in connectivity between the DLPFC and both high and low-level areas, including motor, visual and MDN regions.
Keywords: Autism spectrum disorders; Dorsolateral prefrontal cortex; Neurofeedback; Rt-fMRI.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures





Similar articles
-
Pharmacological intervention for irritability, aggression, and self-injury in autism spectrum disorder (ASD).Cochrane Database Syst Rev. 2023 Oct 9;10(10):CD011769. doi: 10.1002/14651858.CD011769.pub2. Cochrane Database Syst Rev. 2023. PMID: 37811711 Free PMC article.
-
Selecting an optimal real-time fMRI neurofeedback method for alcohol craving control training.Psychophysiology. 2023 Nov;60(11):e14367. doi: 10.1111/psyp.14367. Epub 2023 Jun 16. Psychophysiology. 2023. PMID: 37326428 Free PMC article.
-
Memantine for autism spectrum disorder.Cochrane Database Syst Rev. 2022 Aug 25;8(8):CD013845. doi: 10.1002/14651858.CD013845.pub2. Cochrane Database Syst Rev. 2022. PMID: 36006807 Free PMC article.
-
Behavioural and cognitive behavioural therapy for obsessive compulsive disorder (OCD) in individuals with autism spectrum disorder (ASD).Cochrane Database Syst Rev. 2021 Sep 3;9(9):CD013173. doi: 10.1002/14651858.CD013173.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693989 Free PMC article.
-
Methylphenidate for children and adolescents with autism spectrum disorder.Cochrane Database Syst Rev. 2017 Nov 21;11(11):CD011144. doi: 10.1002/14651858.CD011144.pub2. Cochrane Database Syst Rev. 2017. PMID: 29159857 Free PMC article.
Cited by
-
Neurofeedback technique for treating male schizophrenia patients with impulsive behavior: a randomized controlled study.Front Psychiatry. 2024 Oct 7;15:1472671. doi: 10.3389/fpsyt.2024.1472671. eCollection 2024. Front Psychiatry. 2024. PMID: 39435128 Free PMC article.
-
Elevated Activity in Left Homologous Music Circuits Is Inhibitory for Music Perception but Mediated by Structure-Function Coupling.CNS Neurosci Ther. 2024 Dec;30(12):e70174. doi: 10.1111/cns.70174. CNS Neurosci Ther. 2024. PMID: 39725651 Free PMC article.
-
Neuroplasticity-Based Approaches to Sensory Processing Alterations in Autism Spectrum Disorder.Int J Mol Sci. 2025 Jul 23;26(15):7102. doi: 10.3390/ijms26157102. Int J Mol Sci. 2025. PMID: 40806233 Free PMC article. Review.
References
-
- Hill EL. Evaluating the theory of executive dysfunction in autism. Develop Rev. 2004;24(2):189–233. doi: 10.1016/j.dr.2004.01.001. - DOI
-
- Wang Y, Zhang YB, Liu LL, Cui JF, Wang J, Shum DH, et al. A Meta-Analysis of Working Memory Impairments in Autism Spectrum Disorders. Neuropsychol Rev. 2017;27(1):46–61. 10.1007/s11065-016-9336-y. - PubMed

