Pediatric-type high-grade gliomas with PDGFRA amplification in adult patients with Li-Fraumeni syndrome: clinical and molecular characterization of three cases
- PMID: 38605367
- PMCID: PMC11010357
- DOI: 10.1186/s40478-024-01762-7
Pediatric-type high-grade gliomas with PDGFRA amplification in adult patients with Li-Fraumeni syndrome: clinical and molecular characterization of three cases
Abstract
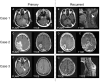
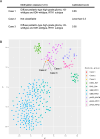
Li-Fraumeni syndrome (LFS) is an autosomal dominant tumor predisposition syndrome caused by heterozygous germline mutations or deletions in the TP53 tumor suppressor gene. Central nervous system tumors, such as choroid plexus tumors, medulloblastomas, and diffuse gliomas, are frequently found in patients with LFS. Although molecular profiles of diffuse gliomas that develop in pediatric patients with LFS have been elucidated, those in adults are limited. Recently, diffuse gliomas have been divided into pediatric- and adult-type gliomas, based on their distinct molecular profiles. In the present study, we investigated the molecular profiles of high-grade gliomas in three adults with LFS. These tumors revealed characteristic histopathological findings of high-grade glioma or glioblastoma and harbored wild-type IDH1/2 according to whole exome sequencing (WES). However, these tumors did not exhibit the key molecular alterations of glioblastoma, IDH-wildtype such as TERT promoter mutation, EGFR amplification, or chromosome 7 gain and 10 loss. Although WES revealed no other characteristic gene mutations or copy number alterations in high-grade gliomas, such as those in histone H3 genes, PDGFRA amplification was found in all three cases together with uniparental disomy of chromosome 17p, where the TP53 gene is located. DNA methylation analyses revealed that all tumors exhibited DNA methylation profiles similar to those of pediatric-type high-grade glioma H3-wildtype and IDH-wildtype (pHGG H3-/IDH-wt), RTK1 subtype. These data suggest that high-grade gliomas developed in adult patients with LFS may be involved in pHGG H3-/IDH-wt. PDGFRA and homozygous alterations in TP53 may play pivotal roles in the development of this type of glioma in adult patients with LFS.
Keywords: PDGFRA amplification; H3-wildtype and IDH-wildtype; Li-Fraumeni syndrome; Pediatric-type high-grade glioma.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

