Chitin-mediated blockade of chitinase-like proteins reduces tumor immunosuppression, inhibits lymphatic metastasis and enhances anti-PD-1 efficacy in complementary TNBC models
- PMID: 38605414
- PMCID: PMC11007917
- DOI: 10.1186/s13058-024-01815-8
Chitin-mediated blockade of chitinase-like proteins reduces tumor immunosuppression, inhibits lymphatic metastasis and enhances anti-PD-1 efficacy in complementary TNBC models
Abstract
Background: Chitinase-like proteins (CLPs) play a key role in immunosuppression under inflammatory conditions such as cancer. CLPs are enzymatically inactive and become neutralized upon binding of their natural ligand chitin, potentially reducing CLP-driven immunosuppression. We investigated the efficacy of chitin treatment in the context of triple-negative breast cancer (TNBC) using complementary mouse models. We also evaluated the immunomodulatory influence of chitin on immune checkpoint blockade (ICB) and compared its efficacy as general CLP blocker with blockade of a single CLP, i.e. chitinase 3-like 1 (CHI3L1).
Methods: Female BALB/c mice were intraductally injected with luciferase-expressing 4T1 or 66cl4 cells and systemically treated with chitin in combination with or without anti-programmed death (PD)-1 ICB. For single CLP blockade, tumor-bearing mice were treated with anti-CHI3L1 antibodies. Metastatic progression was monitored through bioluminescence imaging. Immune cell changes in primary tumors and lymphoid organs (i.e. axillary lymph nodes and spleen) were investigated through flow cytometry, immunohistochemistry, cytokine profiling and RNA-sequencing. CHI3L1-stimulated RAW264.7 macrophages were subjected to 2D lymphatic endothelial cell adhesion and 3D lymphatic integration in vitro assays for studying macrophage-mediated lymphatic remodeling.
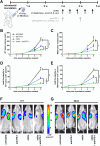
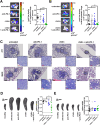
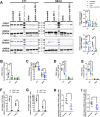
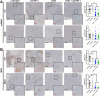
Results: Chitin significantly reduced primary tumor progression in the 4T1-based model by decreasing the high production of CLPs that originate from tumor-associated neutrophils (TANs) and Stat3 signaling, prominently affecting the CHI3L1 and CHI3L3 primary tumor levels. It reduced immunosuppressive cell types and increased anti-tumorigenic T-cells in primary tumors as well as axillary lymph nodes. Chitin also significantly reduced CHI3L3 primary tumor levels and immunosuppression in the 66cl4-based model. Compared to anti-CHI3L1, chitin enhanced primary tumor growth reduction and anti-tumorigenicity. Both treatments equally inhibited lymphatic adhesion and integration of macrophages, thereby hampering lymphatic tumor cell spreading. Upon ICB combination therapy, chitin alleviated anti-PD-1 resistance in both TNBC models, providing a significant add-on reduction in primary tumor and lung metastatic growth compared to chitin monotherapy. These add-on effects occurred through additional increase in CD8α+ T-cell infiltration and activation in primary tumor and lymphoid organs.
Conclusions: Chitin, as a general CLP blocker, reduces CLP production, enhances anti-tumor immunity as well as ICB responses, supporting its potential clinical relevance in immunosuppressed TNBC patients.
Keywords: Chitin; Chitinase-like proteins; Immunosuppression; Immunotherapy; Lymphatic metastasis; Triple-negative breast cancer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Anti-inflammatory signaling by mammary tumor cells mediates prometastatic macrophage polarization in an innovative intraductal mouse model for triple-negative breast cancer.J Exp Clin Cancer Res. 2018 Aug 15;37(1):191. doi: 10.1186/s13046-018-0860-x. J Exp Clin Cancer Res. 2018. PMID: 30111338 Free PMC article.
-
RX-5902, a novel β-catenin modulator, potentiates the efficacy of immune checkpoint inhibitors in preclinical models of triple-negative breast Cancer.BMC Cancer. 2020 Nov 4;20(1):1063. doi: 10.1186/s12885-020-07500-1. BMC Cancer. 2020. PMID: 33148223 Free PMC article.
-
Comparative Profiling of Metastatic 4T1- vs. Non-metastatic Py230-Based Mammary Tumors in an Intraductal Model for Triple-Negative Breast Cancer.Front Immunol. 2019 Dec 17;10:2928. doi: 10.3389/fimmu.2019.02928. eCollection 2019. Front Immunol. 2019. PMID: 31921184 Free PMC article.
-
Biomarkers of Immune Checkpoint Blockade Response in Triple-Negative Breast Cancer.Curr Treat Options Oncol. 2021 Mar 20;22(5):38. doi: 10.1007/s11864-021-00833-4. Curr Treat Options Oncol. 2021. PMID: 33743085 Review.
-
Potential role of chitinase 3-like-1 in inflammation-associated carcinogenic changes of epithelial cells.World J Gastroenterol. 2009 Nov 14;15(42):5249-59. doi: 10.3748/wjg.15.5249. World J Gastroenterol. 2009. PMID: 19908331 Free PMC article. Review.
Cited by
-
Human chitinases and chitinase-like proteins as emerging drug targets - a medicinal chemistry perspective.RSC Med Chem. 2025 Apr 24;16(6):2388-2402. doi: 10.1039/d4md01050g. eCollection 2025 Jun 18. RSC Med Chem. 2025. PMID: 40313579 Free PMC article. Review.
References
-
- Gupta GK, Collier AL, Lee D, Hoefer RA, Zheleva V, Siewertsz van Reesema LL, Tang-Tan AM, Guye ML, Chang DZ, Winston JS, Samli B, Jansen RJ, Petricoin EF, Goetz MP, Bear HD, Tang AH. Perspectives on triple-negative breast cancer: current treatment strategies, unmet needs, and potential targets for future therapies. Cancers Basel. 2020;12(9):2392. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

