Toxicity, physiological response, and biosorption mechanism of Dunaliella salina to copper, lead, and cadmium
- PMID: 38605709
- PMCID: PMC11007151
- DOI: 10.3389/fmicb.2024.1374275
Toxicity, physiological response, and biosorption mechanism of Dunaliella salina to copper, lead, and cadmium
Abstract
Background: Heavy metal pollution has become a global problem, which urgently needed to be solved owing to its severe threat to water ecosystems and human health. Thus, the exploration and development of a simple, cost-effective and environmental-friendly technique to remove metal elements from contaminated water is of great importance. Algae are a kind of photosynthetic autotroph and exhibit excellent bioadsorption capacities, making them suitable for wastewater treatment.
Methods: The effects of heavy metals (copper, lead and cadmium) on the growth, biomolecules accumulation, metabolic responses and antioxidant response of Dunaliella salina were investigated. Moreover, the Box-Behnken design (BBD) in response surface methodology (RSM) was used to optimize the biosorption capacity, and FT-IR was performed to explore the biosorption mechanism of D. salina on multiple heavy metals.
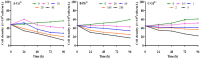
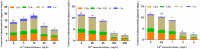
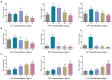
Results: The growth of D. salina cells was significantly inhibited and the contents of intracellular photosynthetic pigments, polysaccharides and proteins were obviously reduced under different concentrations of Cu2+, Pb2+ and Cd2+, and the EC50 values were 18.14 mg/L, 160.37 mg/L and 3.32 mg/L at 72 h, respectively. Besides, the activities of antioxidant enzyme SOD and CAT in D. salina first increased, and then descended with increasing concentration of three metal ions, while MDA contents elevated continuously. Moreover, D. salina exhibited an excellent removal efficacy on three heavy metals. BBD assay revealed that the maximal removal rates for Cu2+, Pb2+, and Cd2+ were 88.9%, 87.2% and 72.9%, respectively under optimal adsorption conditions of pH 5-6, temperature 20-30°C, and adsorption time 6 h. Both surface biosorption and intracellular bioaccumulation mechanisms are involved in metal ions removal of D. salina. FT-IR spectrum exhibited the main functional groups including carboxyl (-COOH), hydroxyl (-OH), amino (-NH2), phosphate (-P=O) and sulfate (-S=O) are closely associated with the biosorption or removal of heavy metalsions.
Discussion: Attributing to the brilliant biosorption capacity, Dunaliella salina may be developed to be an excellent adsorbent for heavy metals.
Keywords: Dunaliella salina; antioxidant; biosorption; heavy metals; physiological response.
Copyright © 2024 Gao, Ling, Tian, Guo and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Al-Homaidan A.A., Al-Houri H.J., Al-Hazzani A.A., Elgaaly G., Moubayed N.M., (2014). Biosorption of copper ions from aqueous solutions by Spirulina platensis biomass. Arab. J. Chem. 7, 57–62. doi: 10. 1016/j.arabjc.2013.05.022, doi: 10.1016/j.arabjc.2013.05.022 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

