One-step and Rapid Identification of SARS-CoV-2 using Real-Time Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP)
- PMID: 38605744
- PMCID: PMC11005395
- DOI: 10.18502/ajmb.v16i1.14165
One-step and Rapid Identification of SARS-CoV-2 using Real-Time Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP)
Abstract
Background: SARS-CoV-2 as the cause of novel coronavirus disease (COVID-19) is a member of the family Coronaviridea that has generated an emerging global health concern. Controlling and preventing the spread of the disease requires a simple, portable, and rapid diagnostic method. Today, a standard method for detecting SARS-CoV-2 is quantitative real-time reverse transcription PCR, which is time-consuming and needs an advanced device. The aim of this study was to evaluate a faster and more cost-effective field-based testing method at the point of risk. We utilized a one-step RT-LAMP assay and developed, for the first time, a simple and rapid screening detection assay targeting the Envelope (E) gene, using specific primers.
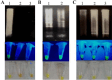
Methods: For this, the total RNA was extracted from respiratory samples of COVID-19 infected patients and applied to one-step a RT-LAMP reaction. The LAMP products were visualized using green fluorescence (SYBR Green I). Sensitivity testing was conducted using different concentrations of the designed recombinant plasmid (TA-E) as positive control constructs. Additionally, selectivity testing was performed using the influenza H1N1 genome. Finally, the results were compared using with conventional real time RT-PCR.
Results: It was shown that the RT-LAMP assay has a sensitivity of approximately 15 ng for the E gene of SARS-CoV-2 when using extracted total RNA. Additionally, a sensitivity of 112 pg was achieved when using an artificially prepared TA-E plasmid. Accordingly, for the detection of SARS-CoV-2 infection, the RT-LAMP had high sensitivity and specificity and also could be an alternative method for real-time RT-PCR.
Conclusion: Overall, this method can be used as a portable, rapid, and easy method for detecting SARS-CoV-2 in the field and clinical laboratories.
Keywords: COVID-19; Detection; LAMP Assay; Real time PCR; SARS-CoV-2.
Copyright© 2024 Avicenna Research Institute.
Figures



Similar articles
-
A Multiplex and Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Sensitive and Rapid Detection of Novel SARS-CoV-2.Front Cell Infect Microbiol. 2021 Jun 29;11:653616. doi: 10.3389/fcimb.2021.653616. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34268131 Free PMC article.
-
Development and Validation of a Novel COVID-19 nsp8 One-Tube RT-LAMP-CRISPR Assay for SARS-CoV-2 Diagnosis.Microbiol Spectr. 2022 Dec 21;10(6):e0196222. doi: 10.1128/spectrum.01962-22. Epub 2022 Nov 29. Microbiol Spectr. 2022. PMID: 36445095 Free PMC article.
-
A New Method to Detect Variants of SARS-CoV-2 Using Reverse Transcription Loop-Mediated Isothermal Amplification Combined with a Bioluminescent Assay in Real Time (RT-LAMP-BART).Int J Mol Sci. 2023 Jun 27;24(13):10698. doi: 10.3390/ijms241310698. Int J Mol Sci. 2023. PMID: 37445876 Free PMC article.
-
Reverse Transcriptase Loop Mediated Isothermal Amplification (RT-LAMP) for COVID-19 diagnosis: a systematic review and meta-analysis.Pathog Glob Health. 2021 Jul;115(5):281-291. doi: 10.1080/20477724.2021.1933335. Epub 2021 Jun 4. Pathog Glob Health. 2021. PMID: 34086539 Free PMC article.
-
Nanomaterials to tackle the COVID-19 pandemic.Emergent Mater. 2021;4(1):211-229. doi: 10.1007/s42247-021-00184-8. Epub 2021 Feb 12. Emergent Mater. 2021. PMID: 33615139 Free PMC article. Review.
References
-
- Sedighimehr N, Fathi J, Hadi N, Rezaeian ZS. Rehabilitation, a necessity in hospitalized and discharged people infected with COVID-19: a narrative review. Physical Therapy Reviews 2021;26(3):202–10.
LinkOut - more resources
Full Text Sources
Miscellaneous
